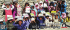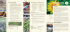0974-7893 (Print) - Journal of Threatened Taxa
Transcription
0974-7893 (Print) - Journal of Threatened Taxa
ISSN
0974-7907 (Online)
0974-7893 (Print)
July 2014
Vol. 6 | No. 8
Pages: 6053–6152
OPEN ACCESS
www.threatenedtaxa.org
ate of P blica ion
ly
nline Print
DOI: 10.11609/JoTT.26jul14.60 -6152
ISSN: 0974-7907 (Online), 0974-7893 (Print)
Published by Wildlife Information Liaison Development Society Typeset and printed at
Zoo Outreach Organization
96, Kumudham Nagar, Vilankurichi Road, Coimbatore, Tamil Nadu 641035, India
Ph: +91 422 2665298, 2665101, 2665450; Fax: +91 422 2665472
Email: threatenedtaxa@gmail.com, articlesubmission@threatenedtaxa.org
www.threatenedtaxa.org
EDITORS
Founder & Chief Editor
Dr. Sanjay Molur, Coimbatore, India
Managing Editor
Mr. B. Ravichandran, Coimbatore, India
Associate Editors
Dr. B.A. Daniel, Coimbatore, India
Dr. Manju Siliwal, Dehra Dun, India
Dr. Meena Venkataraman, Mumbai, India
Ms. Priyanka Iyer, Coimbatore, India
Editorial Advisors
Ms. Sally Walker, Coimbatore, India
Dr. Robert C. Lacy, Minnesota, USA
Dr. Russel Mittermeier, Virginia, USA
Dr. Thomas Husband, Rhode Island, USA
Dr. Jacob V. Cheeran, Thrissur, India
Prof. Dr. Mewa Singh, Mysuru, India
Dr. Ulrich Streicher, Oudomsouk, Laos
Mr. Stephen D. Nash, Stony Brook, USA
Dr. Fred Pluthero, Toronto, Canada
Dr. Martin Fisher, Cambridge, UK
Dr. Ulf Gärdenfors, Uppsala, Sweden
Dr. John Fellowes, Hong Kong
Dr. Philip S. Miller, Minnesota, USA
Prof. Dr. Mirco Solé, Brazil
Editorial Board
Subject Editors 2013–2014
A.J. Solomon Raju, Andhra University, Visakhapatnam, India
Albert G. Orr, Griffith University, Nathan, Australia
Alexander Ereskovsky, IMBE, Marseille, France
Anjana Silva, Rajarata University of Sri Lanka, Saliyapura, Sri Lanka
Annemarie Ohler, Muséum national d’Histoire naturelle, Paris, France
Ansie Dippenaar-Schoeman, University of Pretoria, Queenswood, South Africa
Antonio A. Mignucci-Giannoni, Universidad Interamericana de Puerto Rico, Puerto Rico
Anwaruddin Chowdhury, Guwahati, India
Aparna Watve, Pune, Maharashtra, India
Arthur Y.C. Chung, Sabah Forestry Department, Sandakan, Sabah, Malaysia
B. Ravi Prasad Rao, Sri Krishnadevaraya University, Anantpur, India
B. Shivaraju, Bengaluru, Karnataka, India
B.A. Daniel, Zoo Outreach Organization, Coimbatore, Tamil Nadu, India
B.S. Kholia, Botanical Survey of India, Gangtok, Sikkim, India
Brett C. Ratcliffe, University of Nebraska, Lincoln, USA
Brian Fisher, California Academy of Sciences, USA
C. Raghunathan, Zoological Survey of India, Andaman and Nicobar Islands
C. Srinivasulu, Osmania University, Hyderabad, India
Carl Ferraris, Smithsonian Institution, Portland, USA
Cecília Kierulff, Victorville , California
Chris Bowden, Royal Society for the Protection of Birds, Sandy, UK
Christoph Kueffer, Institute of Integrative Biology, Zürich, Switzerland
Christoph Schwitzer, University of the West of England, Clifton, Bristol, BS8 3HA
Cleofas Cervancia, Univ. of Philippines Los Baños College Laguna, Philippines
Colin Groves, Australian National University, Canberra, Australia
Crawford Prentice, Nature Management Services, Jalan, Malaysia
D.B. Bastawade, Maharashtra, India
D.J. Bhat, Retd. Professor, Goa University, Goa, India
Dale R. Calder, Royal Ontaro Museum, Toronto, Ontario, Canada
Daniel Brito, Federal University of Goiás, Goiânia, Brazil
David Mallon, Zoological Society of London, UK
Davor Zanella, University of Zagreb, Zagreb, Croatia
Deepak Apte, Bombay Natural Hisotry Society, Mumbai, India.
Dietmar Zinner, German Primate Center, Göttingen, Germany
E. Vivekanandan, Central Marine Fisheries Research Institute, Chennai, India
Eduard Vives, Museu de Ciències Naturals de Barcelona, Terrassa, Spain
Eric Smith, University of Texas, Arlington, USA
Erin Wessling, Max Planck Institute for Evolutionary Anthropology, Germany
F.B. Vincent Florens, University of Mauritius, Mauritius
Ferdinando Boero, Università del Salento, Lecce, Italy
Francesco Dal Grande, Senckenberg Gesellschaft für Naturforschung, Frankfurt
George Mathew, Kerala Forest Research Institute, Peechi, India
Gernot Vogel, Heidelberg, Germany
Giovanni Amori, CNR - Institute of Ecosystem Studies, Rome, Italy
Gombobaatar Sundev, Professor of Ornithology, Ulaanbaatar, Mongolia
G.P. Sinha, Botanical Survey of India, Allahabad, India
H.C. Nagaveni, Institute of Wood Science and Technology, Bengaluru, India
H.C. Paulo Corgosinho, Bairro Universitário, Frutal, Brazil
Heidi S. Riddle, Riddle’s Elephant and Wildlife Sanctuary, Arkansas, USA
Hem Sagar Baral, Charles Sturt University, NSW Australia
Hemant V. Ghate, Modern College, Pune, India
Heok Hee Ng, National University of Singapore, Science Drive, Singapore
Hui Xiao, Chinese Academy of Sciences, Chaoyang, China
Ian J. Kitching, Natural History Museum, Cromwell Road, UK
Ian Redmond, UNEP Convention on Migratory Species, Lansdown, UK
Indraneil Das, Sarawak, Malaysia
J. Jerald Wilson, King Abdulaziz University, Jeddah, Saudi Arabia
J.W. Duckworth, IUCN SSC, Bath, UK
Jack Tordoff, Critical Ecosystem Partnership Fund, Arlington, USA
James Young, Hong Kong Lepidopterists’ Society, Hong Kong
Jeff McNeely, IUCN, Gland, Switzerland
Jesse Leland, Southern Cross University, New South Wales, Australia
Jill Pruetz, Iowa State University, Ames, USA
Jodi L. Sedlock, Lawrence University, Appleton, USA
John C. Morse, Clemson University, Long Hall, Clemson, USA
John Noyes, Natural History Museum, London, UK
John Veron, Coral Reef Foundation, Townsville, Australia
K. Ravikumar, FRLHT, Bengaluru, Karnataka, India
K.A. Subramanian, Zoological Survey of India, New Alipore, Kolkata, India
K.G. Sivaramakrishnan, Madras Christian College, Chennai, Tamil Nadu, India
K.S. Gopi Sundar, International Crane Foundation, Baraboo, USA
K.S. Negi, NBPGR-ICAR, Nainital District, Uttarakhand, India
Kailash Chandra, Zoological Survey of India, Jabalpur, Madhya Pradesh, India
Karin Schwartz, George Mason University, Fairfax, Virginia.
Kevin Smith, IUCN, Cambridge, UK
Klaus Ruetzler, Smithsonian Institution, Washington, DC
Kristin Leus, Copenhagen Zoo, Annuntiatenstraat, Merksem, Belgium
Kumaran Sathasivam, Marine Mammal Conservation Network of India, India
Lala A.K. Singh, Bhubaneswar, Orissa, India
Late Dr. T.C. Narendran, (Retired) Professor, University of Calicut, Kerala, India
Llewellyn D. Densmore, Texas Tech University, Lubbock, USA
Lukas Rüber, Department of Vertebrates, Natural History Museum, Switzerland
M. Afzal Khan, Department of Zoology, Aligarh Muslim University, Aligarh, India
Mandar S. Paingankar, University of Pune, Pune, Maharashtra, India
Manju Siliwal, WILD, Coimbatore, Tamil Nadu, India
Martin B.D. Stiewe, The Natural History Museum, UK
Meena Venkataraman, Mumbai, India
Merlin Franco, Curtin University, Malaysia
Mewa Singh, Mysore University, Mysore, India
Mohammad Hayat, Aligarh Muslim University, Aligarh, India
Mohilal Meitei, Manipur University, Camchipur, Manipur, India
N.P. Balakrishnan, Ret. Joint Director, BSI, Coimbatore, India
continued on the back inside cover
Front cover: Flowers and fruit of Sloanea sterculiacea, and Canarium strictum tree in the background. Artwork by Pitchandikulam Forest Consultats.
See Ravikumar et al. 6108–6121, and Kulkarni et al. 6093–6100
o rnal of hreatened a a
.threatenedta a.or
P
M. afar l slam Moayyad her hah
ly
M
A
A
ISSN
Online 0974–7907
Print 0974–7893
Ahmed o
National Wildlife Research Center, PO Box 1086, Taif, Saudi Arabia AND Saudi Wildlife Authority, PO Box 61681,
Riyadh 11575, Saudi Arabia
1
mzafarul.islam gmail.com (corresponding author)
1,2,3
P
A
Abstract The Arabian Gazelle is a globally threatened antelope (Vulnerable) in Saudi Arabia. Small relict populations remain in limited
areas, while historically Arabian gazelles occurred in Mahazat as-Sayd protected area in central Saudi Arabia but were exterminated by
anthropogenic and other pressures, including habitat loss and hunting. Important habitat has been lost to agricultural developments, fencing
of pasture for livestock and the construction of human settlements and roads. The reintroduction of Arabian Gazelles was undertaken in
Mahazat during 2011–2014 to bring back this locally extinct species study its ecology and biology in a fenced protected area. We released a
total of 49 (12 males, 37 females) animals. A year after release animals started breeding and six calves have been recorded so far with more
to come. The gazelles prefer to use more rocky areas where shrubs and acacia trees occur in the reserve, and do not move long distances
except for one individual that moved more than 50km. Mahazat is fenced, which prevents local people from entering the reserve to poach
or otherwise disturb animals. Management lessons include the need for continued monitoring of reintroduced populations. Interactions
between Arabian and Sand Gazelles ( a ella subgutturosa marica) and Arabian Oryx (Oryx leucoryx) were also studied.
ey ords Arabia, gazelles, management, reintroduction, threatened.
http://dx.doi.org/10.11609/JoTT.o3971.6053-60 | oo an
urn:lsid:zoobank.org:pub:631E39DC-623C-4B68-A94C-04DE65C3D46E
ditor David Mallon, Zoological Society of London, UK.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3971 | Received 16 March 2014 | Final received 05 May 2014 | Finally accepted 24 June 2014
itation Islam, M.Z., M.S. Shah & A. Boug (2014). Re-introduction of globally threatened Arabian Gazelles Gazella arabica (Pallas, 1766) (Mammalia: Bovidae) in
fenced protected area in central Saudi Arabia. Journal of Threatened Taxa 6(8): 6053–6060; http://dx.doi.org/10.11609/JoTT.o3971.6053-60
o yri ht © Islam 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and
distribution by providing adequate credit to the authors and the source of publication.
ndin The Project is funded by the Saudi Wildlife Authority, Riyadh Saudi Arabia.
om etin nterest The authors declare no competing interests.
A thor ontrib tion All authors have been involved in designing and making the project proposal till reintroduction and animals monitoring in Mahazat.
A thor etails D . M. Z
-U I
is Research Director and field biologist with strong interest in international wildlife conservation and had been associated
with BirdLife International (UK), RSPB (UK), BNHS (India) and Aligarh Muslim University (India). His main research work is on ecology and biology of threatened
taxa with their habitat evaluation and niche modelling. Since April 2006, he is looking after the re-introduction and other field research programs of the country
with the National Wildlife Research Center in Taif, Saudi Arabia. M . M
S
S . is field researcher working in Mahazat as-Sayd protected area in Saudi
Arabia since last 14 years on Houbara Bustard and also worked on Arabian and Sand gazelles, and Sand Cat. M . A
B
is General Director of National
Wildlife Research Center and field biologist who studied the ecology and biology of Hamadrays Baboon in Saudi Arabia. He has produced several papers in
international journals on his research.
Ac no led ements We want to extend our thanks and gratitude to HH Prince Bander Bin Saud Bin Mohammed Al Saud (President, Saudi Wildlife Authority)
for his leadership, generosity and continuous support towards the research and conservation work by the NWRC in the Kingdom. All NWRC/KKWRC staffs are
acknowledged for their help, especially Dr. Mohammed Sandouka, Raziman Khan, Mr. Sham Davande for help in mapping and Ms. Parveen Khan (Librarian) for
providing papers and literature during writing this paper. We want to thank Dr. David Mallon for reviewing this paper.
e introd ction of Arabian a elles in central a di Arabia
slam et al.
The taxonomy of gazelles is notoriously complex,
and several classifications have been proposed. Three
species have been reported from Saudi Arabia: Saudi
Gazelle Gazella saudiya (now extinct), Mountain Gazelle
Gazella gazella (widespread in the Arabian Peninsula)
and Arabian Gazelle Gazella arabica (known only from a
specimen collected in the 1820s on the Farasan Islands
in the Red Sea). Recent genetic research has questioned
this arrangement. Lerp et al. (2013) indicated that G.
gazella in fact consists of two clades, one in the north
centred on the Golan Heights, and a southern clade
covering the rest of the former range. B rmann et
al. (2012) demonstrated that G. arabica had been
misidentified and the specimen was in fact G. gazella.
These authors, therefore, proposed a nomenclatural
change, preferring to use the name G. arabica for
the southern clade of G. gazella. This arrangement is
followed here for gazelles in Saudi Arabia, including the
former subspecies G.g. cora and G.g. farasani (Thouless
& Bassri 1991; Groves 1997; Flammand et al. 1998;
Grubb 2005).
The former range of G. gazella included southern
Turkey, Israel, Iran (Farur Island), Oman, United Arab
Emirates, Yemen and Saudi Arabia (Mallon & Kingswood
2001; Kank l et al. 2012). Occasional sightings have
been reported from Egypt (Sinai), Syria, Lebanon and
Jordan (Kingswood & Khairallah 2001; Saleh 2001;
Masseti 2004). The range of G. g. cora (here referred
to as the Arabian Gazelle G. arabica) occurred across
most of the Arabian Peninsula from the Arava Valley in
southern Israel, along the Hejaz and Asir Mountains in
western Saudi Arabia (12-28 N), through Yemen, Oman
and into the Emirates (Shalmon 1987; Uerpmann 1987;
Dunham et al. 2001; Insall 2001; Mallon & Al-Safadi
2001; Samour 2001).
In Saudi Arabia, Arabian Gazelle numbers have
decreased dramatically throughout their range since
the middle of the 20th century due to anthropogenic
and other pressures including habitat loss and hunting.
Important habitat has been lost to agricultural
developments, fencing of pasture for livestock and the
construction of human settlements and roads (Habibi
1986; Nader 1989; Thouless et al. 1991; Islam et al.
2010a,b). The IUCN Red List (IUCN 2009, 2014) currently
ranks this species (as G. gazella) as Vulnerable’ (A2ad).
Small relict populations may still occur in Al Khunfah and
Harrat al Harrah in the north of Saudi Arabia (Harrison
1968; Green 1986; Thouless et al. 1991; Habibi 1992;
Wacher 1993; Seddon et al. 1997; Islam et al. 2012) and
on the Tihama coastal plain (Thouless et al. 1991, 1997;
Magin 1993, 1996; Wacher & AlAgeel 2001a; Ahmed
Boug pers. comm. March 1999, in Wadi Hali; Zafar-ul
Islam pers. obs. 22 February 2009, 80km south of Al
unfidah). On the Farasan Islands a strong population
of about 1000 individuals has survived (Flammand et
al. 1998; Cunningham & Wronski 2010), and Arabian
Gazelles were released in two protected areas (Ibex
Reserve, Uruq Bani Ma’Arid) from 1990 to 2007 (Islam
et al. 2012; Wronski et al. 2012a,b,c). Most records
of natural Arabian gazelle populations in Saudi Arabia
originate from the western part of the Kingdom, i.e., the
Asir, Sarawat and Hejaz Mountains (Thouless et al. 1991,
1997; Magin 1993, 1996; Al-Hazmi & Ghandour 1992;
Magin & Greth 1994). Four populations are known from
the northern Hejaz Mountains extending from Medina
up towards the Gulf of Aqaba, namely Jibal Kallab,
Harrat Uwayrid, Ras Suwaihil and Jibal Dakhkhan (Child
& Grainger 1990; Thouless et al. 1991, 1997; Wacher &
AlAgeel 1999; Wacher & Strauss 2000; Wacher 2001;
Ahmed Boug pers. comm. March 1999, near Al Farah).
The Hejaz Mountains are more arid than the southern
Asir and Sarawat Mountains where permanent water is
available throughout the year. In the Asir and Sarawat
Mountains a number of gazelle populations were
reported to survive in the foothills and in the eastern
flanks of the Asir, where they are mostly associated with
Acacia-lined wadis (Thouless et al. 1991). From 1990–
2001, several surveys have focused on a number of
populations in the Asir National Park (Al Khalili & Nader
1984; M.Z. Islam pers. comm. 2013) and other proposed
protected areas in the region to confirm presence or
absence of gazelles or to establish rough population
estimates (Child & Grainger 1990; Magin 1993, 1996;
Thouless et al. 1997; Wacher & Al Toum 1998; Wacher &
AlAgeel 2001b). Since 2001 no confirmed observations
of Arabian Gazelles in the Asir Mountains have been
reported. Three observations were made by a local
shepherd near Jabal Kabkab in Makkah in January 2014
(A. Boug and wildlife rangers pers. comm. 2014).
Historically, Arabian Gazelles occured in Mahazat
as-Sayd Protected Area in central Saudi Arabia but
their loss was attributed to anthropogenic and other
pressures (Child & Grainger 1990; Lewlin in press, Islam
et al. 2010a,b). Since their presence was confirmed
via interviews with local people and historical records
(Magin 1996; Thouless et al. 1997; Islam et al. 2010a,b),
the Strategy and Action Plan of the National Wildlife
Research Center (NWRC) suggested the re-introduction
of Arabian Gazelles (Islam et al. 2010a,b). This project is
particularly significant as it is one of the first successful
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
e introd ction of Arabian a elles in central a di Arabia
releases for the species in over 20 years (Islam et al.
2011, 2012). After many years of dedicated work to
identify and conserve different species of gazelles in
Saudi Arabia, these elegant gazelles were successfully
released. The release is part of the ongoing efforts
in the Kingdom to conserve a variety of antelopes, an
initiative that is strongly supported by the Saudi people
(Magin 1996; Thouless et al. 1997; Islam et al. 2010a,b).
The need to reintroduce gazelles from captive bred
populations was identified at an early stage by the Saudi
Wildlife Authority and endorsed by an International
Conference on Conservation of Arabian Gazelles (Greth
et al. 1996). In line with conference recommendations,
the IUCN guidelines for reintroduction were followed
(IUCN 1998). Key subsidiary recommendations in this
approach to reintroduction are that only appropriate
taxa are released within their former historic range and
that the original cause of extinction has been identified
and controlled or eliminated. Gazelles reintroduced
have all been placed into former range areas in fenced
protected area. Extensive taxonomic research, including
molecular genetic research, has been undertaken to
ensure accurate identification in this di cult group
(Greth et al. 1996; Hammond et al. 2001).
The reintroduction of the Arabian Gazelles was
undertaking with the following goals: (a) to re-establish
wild and self-sustaining populations of Arabian Gazelle
in Saudi Arabia; (b) to study the most suitable habitats
and establish protected areas in which vegetation can
recover; (c) to manage the re-introduction of herds in
protected areas; (d) to re-introduce in suitable habitats;
and (e) to study the ecology and biology of gazelles in
the protected area.
A
A
The
Mahazat
as-Sayd
Protected
Area
(22015’N–41040’E) is located in west-central Saudi
Arabia and consists of a gently undulating sand and
gravel plain at about 900m altitude comprising dwarfscrubland dominated by Acacia tortilis trees, other
Acacia ehrenbergiana as well as Maerua crassifolia trees
(Fisher & Membery 1998). Perennial grasses, such as
Panicum turgidum, Lasiurus scindicus and Octhochloa
ompressa, which are important Arabian Gazelle forage
species (Blank 2000; Shah et al. 2013), are abundant
on sandy areas and some of elevated areas and
depressions. Net primary production in Mahazat asSayd is low and rainfall is unpredictable and patchily
distributed (Treydte et al. 2001). Mean temperature
slam et al.
ranges from 17 C in winter to 340C in summer, but
maximum temperatures in summer often exceed 450C
(Islam et al. 2012). Mahazat as-Sayd is completely fenced
(2244km2) and was gazetted in 1988 as a reintroduction
site for Arabian Oryx (Oryx leucoryx), Houbara Bustard
(Chlamydotis mac ueenii) and the Arabian Sand Gazelle
( a ella subgutturosa marica) and Arabian Gazelle
(Vessey-Fitzgerald 1952; Child & Grainger 1990; Islam
et al. 2012). Predators were originally eradicated from
several protected areas, but one Arabian Wolf (Canis
lupus) was located in Mahazat in 2008 (Musleh Ammar
(Head ranger) pers. comm. 2012). The Arabian Gazelles
were originally extirpated primarily by excessive hunting
before declaration of PA (Islam et al. 2010a,b).
After the identification the area as wildlife reserve
with fencing and proper protection from livestock
grazing, within five years the recovery of the vegetation
increased the chances of re-introduction of Arabian
Gazelles in the area as compared to areas outside the
reserve, which was overgrazed and disturbed. The
local community was taken in to confidence during the
process and Saudi Wildlife Authority got full support
both from civil society and the government for the reintroduction of native wildlife.
M
Arabian Gazelles were captured just before dark
by using boma-trapping and put in individual crates
constructed of plywood and measuring 100x36x90
cm. Crates could be opened from both ends and had
30-40 ventilation holes of 1cm diameter. Animals were
transported the 670km to Mahazat at night by truck.
Upon arrival at the reserve the gazelles were placed in a
quarantine enclosures identical in size (500x500 m) and
features to those at the KKWRC. Food and water were
provided in enclosure. Arabian Gazelles were obtained
from King Khalid Wildlife Research Center (KKWRC).
All the translocated gazelles were born in captivity at
KKWRC.
Between 2011 and 2014, three groups of animals
were released from the pre-release enclosure into
wild when the vegetation condition was favorable. All
animals were released by opening gates of pre-release
enclosure and allowing them to leave of their own, while
water and alfalfa was provided outside of the enclosure
for three weeks. All animals that were radio-tagged
were monitored on a daily basis by ground telemetry
and at least once a fortnight by aerial telemetry using
Maule aircraft and date, time, location, activity, habitat
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
M. Zafar-ul Islam
slam et al.
M. Zafar-ul Islam
e introd ction of Arabian a elles in central a di Arabia
ma e . Arabian Mo ntain a elles ith collars males and a female
M.S. Shah
O. Couppey
ma e . Arabian Mo ntain a elle male in the ni ht
ma e . Arabian Mo ntain a elle male ith collars
ma e . Arabian Mo ntain a elle released by
President of
A
and group compositions were recorded.
First Release: The first group of 17 (4 males, 13
females) Arabian Gazelles was transferred from KKWRC
to Mahazat on 14 March 2011 by road. Age of gazelles
ranged between three months old calf to 10 years old.
Radio-collars were secured to each individual with tag
numbers. One female died on 19 March 2011 in the
release pen. On 08 April 2011 four Arabian Gazelles (1
male, 3 females) were released directly from boxes by
His Highness Prince Bandar bin Saud bin Mohammed Al
Saud (President of SWA), including two (1 male, 1 female)
from NWRC and other two females from KKWRC. The
remaining 14 gazelles (4 males, 10 females) were kept in
the pre-release enclosure and released by opening the
gate (Images 1–4).
Second Release: The second group of 23 (8 males,
15 females) gazelles was transferred from KKWRC to
Mahazat on 12 February 2012. Age structure of this
group was mostly 2–4 years old and ranged between
1–5 years old animals. One female gazelle was recorded
Prince ander
dead in the pre-release enclosure on 21 February 2012.
This group of 22 Arabian Gazelles was released by
opening the enclosure gate on 06 March 2012.
Third release: Another nine animals (2 males; 7
females) were released in Mahazat in June 2014.
All animals were tested for tuberculosis, vaccinated
against rabies, foot and mouth disease, rinderpest,
and pasteurellosis, marked with either eartags, marker
collars, or radio transmitters, and placed in quarantine
pens for a few months and soft released by opening the
gate of the enclosure.
Post-release monitoring: In summer of 2011 and
2014, when the vegetation mostly dried off, a total of
eight Arabian Gazelles were recorded dead, mostly
just after the release from the first release between
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
e introd ction of Arabian a elles in central a di Arabia
slam et al.
Number f AArabian
rabian Gaazelles mberoof
elles
60
60 50
50 40
40 30
30 20
20 10
10 0 0
i
Male
female
a n born a er release
otal
Female Fawn born a4er Total Alive by July 2014 Dead by Jrelease une 2014 Total Released All June 2014 Alive by July 2014
Dead by June 2014
Total released till June 2014
Male re . tat s of re introd ced Arabian a elles in Maha at PA ith fa n born in the ild
May and November 2011 and five gazelles (1 male, 4
females) went missing due to radio-collar failure and the
radio collar fell off one female. These animals were not
recorded again until now. Only one female was found
dead on 13 March 2012 among the second released
group. Mortalities were controlled by further improving
the release method by releasing animals in winter
months, not as late as in 2011. Another factor assisting
the successful release was that the reserve received
good rainfall contributing to the growth of greenery.
Post-release dispersal of Arabian Gazelles has been
recorded from the intensive monitoring programmes.
After release, the productivity was high. After one year
of release, the gazelles started breeding and five radiotagged females gave birth to calves (Fig. 1).
Breeding records of the gazelles: The first wild born
Arabian Gazelles calf was recorded in Mahazat on 28
August 2012 near the fence. This calf was almost one
month old when recorded with the group. Six other
females delivered one each by end of September 2012.
The offspring show more adaptability to the wild than
their captive-bred parents. All young animals born
before the end of 2012 were alive at the end of 2013.
The present population of Arabian Gazelle in Mahazat
reserve is 40–50 (Fig. 1).
Studies related to habitat use, feeding ecology,
range and space use, and group composition are being
carried out in Mahazat.
abitat reference by Arabian a elles
A recent spot image with 2.5m resolution in color
was acquired from the remote sensing section of the
King Abduaziz City for Science and Technology in Saudi
Arabia for the Mahazat as-Sayd Protected Area to study
the landcover and classified in four major classes. The
dominant landcover class is sandy followed by rocky
able . andco er class cation of satellite ima e and habitat se by
a elles
andco er
class
Area in
s m
Percent
Area
Percent Area sed by
Arabian a elle
landco er
1
Sandy areas
1014
45
08
2
Rocky barren
478
21
43
3
Grasslands
432
19
34
4
Scrub forest
320
14
17
2244
100
100
Total
and grassland as depicted in Table 1 and Fig. 2. Arabian
Gazlles use mainly scrub and grassland areas in the rocky
barren and most avoiding sandy areas in the reserves
(Fig. 2).
Food and water: The released gazelles and their
offspring were mixed feeders, eating grasses, forbs, and
shrubs. Growing leaves of Acacia tortilis were eaten.
Occasionally gazelles were observed to stand on their
hind legs, resting the forelegs on bush, to reach leaves
growing above their normal levels.
After release, gazelles were occasionally seen
drinking water from the waterhole that was created
especially for them. Once animals moved away from the
release sites, the territorial male was rarely seen outside
his territory; however, regular checks were made for
the gazelle footprints near the release site and some of
the animals used to visit in the late evening and early
morning in search of food and water.
One of the males moved almost 20km from the
release sites. The release site is near to the hilly part
of the reserve (10km away) and most of the animals
are confined to these areas. Females who were about
to deliver young went to a secluded place and after
delivering the calf, female and the calf moved around
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
e introd ction of Arabian a elles in central a di Arabia
i
slam et al.
re . and se a ern and distrib tion of Arabian a elles in Maha at PA.
50m away, likely to avoid small carnivores being
attracted to the smell of the placenta.
The reintroduction of the Arabian Gazelles in
Mahazat as-Sayd has faced some di culties including
(a) maintaining long-term regular monitoring due to
lack of full time researchers; (b) lack of skills for mass
capture techniques for Arabian Gazelles in case of need
to fix or re-fix the radio collars, especially of new born
individuals.
Major lessons learned from other reintroductions in
Saudi Arabia include (a) when wide-ranging species are
confined to restricted areas, even if such areas are large,
it is essential that an effective population management
plan is in place before any re-introduction is carried
out, and that the plan is properly implemented. If this
is not done, large-scale mortalities will occur (Islam
et al. 2010a,b); (b) prior to any transplantation, range
conditions in the release area have to be improved and
the area protected from livestock exploitation. Once
pasture conditions show adequate signs of improvement
and the site is adequately protected, re-introduction of
the animals can be contemplated; (c) the time of release
should coincide with suitable vegetation conditions; (d)
keeping the animals in pre-release enclosures within
the re-introduction site to get them acclimatized to the
natural environment and provide minimal amount of
food and water; (e) regulate tourism in re-introduction
areas as this can lead to increased habitat degradation;
and (f) a public-awareness program should be put in
place to inform citizens of the biological and historic
significance of the Sand Gazelle in the society.
A healthy (disease-free, injury-free) breeding Arabian
Gazelle population has been established in Mahazat asSayd Protected Area for more than two years, and is
considered to be a success. Productivity by released
Arabian Gazelles high; society and the government
support the re-introduction and Mahazat has been
suggested as a destination for national and international
tourists.
Mana ement lan
The ungulate populations in the Mahazat As-Sayd
Protected Area are valuable resources that could be
put to good conservation (or other use) with careful
planning. An active management plan for the ungulate
populations in Mahazat As-Sayd Protected Area was
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
e introd ction of Arabian a elles in central a di Arabia
developed in the end of 2008 by experts including
ecologists, biologists, botanists, vets, sociologists and
policy and decision makers to minimize periodic largescale mortalities in the reserve (Islam et al. 2010b).
In the plan artificial provision of water and alfalfa
is provided at five different locations to animals in
Mahazat since May 2008 to minimize mortality.
Under this project, auxiliary rangers are given
training to monitor the population and protect it from
suspected hunters. Such surveillance would be greatly
facilitated by the rugged nature of most gazelles in the
area.
Al halili A. .
.A. ader
. Nature conservation in Saudi
Arabia. An ecological study of the Asir National Park with check-list
of the terrestrial vertebrate fauna of the park and its surrounding.
Fauna of Saudi Arabia 6: 11–31.
Al a mi M.A.
A.M. hando r
. An ecological study of
gazelles in the western and southern regions of Saudi Arabia.
Journal of Arid Environments 23: 279–286.
rmann . . .
rner . r enbec
. .
ssner . ebel
.
rheide
. The curious case of Gazella arabica.
ammalian Biology 78: 220–225; http://dx.doi.org/10.1016/j.
mambio.2012.07.003
lan
.A.
. Acacia gazelle increases with habitat improvement.
nusletter 19(1): 11–13.
hild .
. rain er
. A system plan for protected areas for
wildlife conservation and sustainable rural development. National
Commission for Wildlife Conservation and Development, Riyadh.
nnin ham P. .
. rons i
. Twenty years of gazelle
monitoring on Farasan Islands: An overview. Oryx 45(1): 50–55;
http://dx.doi.org/10.1017/S0030605310001298
nnin ham P. . . rons i
. Al A eel
. Predators
persecuted in the Asir Region, western Saudi Arabia. Wildlife Middle
East News 4(1): 6.
nham .M. . . illiamson
. o bert
. Saudi Arabia,
pp. 55–62. In: Mallon, D.P. & S.C. Kingswood (eds). Antelopes, Part
North Africa, the iddle East, and Asia. IUCN Global Survey and
Regional Action Plan, Gland, Switzerland.
isher M.
.A. Membery
. Climate, pp. 5–38. In: Ghazanfar,
S.A. & M. Fisher (eds.). Vegetation of the Arabian Peninsula. Kluwer
Academic Publishers, Dordrecht.
lammand . . . . . ho less . at any
. . Asmod
.
Status of the gazelles of the Farasan Islands, Saudi Arabia. ammalia
52(4): 608–610; http://dx.doi.org/10.1515/mamm-1988-0422
reen A A.
. Status of large mammals in northern Saudi
Arabia.
ammalia 50(4): 483–493; http://dx.doi.org/10.1515/
mamm.1986.50.4.483
reth A. . Ma in
M. Ancrena eds.
. Conservation of
Arabian Gazelles. Proceedings of the Symposium Establishing
Priorities for Gazelle Conservation in the Arabian Peninsula ,
held at the National Commission for Wildlife Conservation and
Development (NCWCD) in Riyadh, Saudi Arabia, 31 October - 03
November 1992, 168pp.
ro es .P.
. Taxonomy of Arabian Gazelles, pp. 24–51. In:
Habibi, K., A. Abu-Zinada & I.A. Nader (eds.). The Gazelles of Arabia.
National Commission for Wildlife Conservation and Development,
Riyadh.
r bb P.
. Artiodactyla, pp. 637–722. In: Wilson, D.E. & D.M.
Reeder (eds.). ammal Species of the orld. A Taxonomic and
slam et al.
eographic eference ( rd Edition). Johns Hopkins University Press,
Baltimore, USA.
abibi
.
. Arabian ungulates: their status and future
protection. Oryx 20: 100–103.
abibi .
. Arabian Gazelles. National Commission for Wildlife
Conservation and Development. Publication No. 4, Riayadh.
arrison . .
. ammals of Arabia - olume . Ernest Benn,
London.
ammond . .
. Macasero . lores
. . Mohammed .
acher
M. r ford
. Phylogenetic reanalysis of the
Saudi Gazelle and its implications for conservation. Conservation
Biology 15(4): 1123–1133; http://dx.doi.org/10.1046/j.15231739.2001.0150041123.x
nsall . .
. Oman, pp. 69–73. In: Mallon, D.P. & S.C. Kingswood
(eds.). Antelopes. Part North Africa, the iddle East, and Asia.
lobal Survey and egional Action Plans. IUCN, Gland, Switzerland.
slam M. . . n tson A. o
b . Strategy and Action Plan
to Reduce the Risk of Mass Mortalities of Reintroduced ungulates in
the Mahazat as-Sayd Protected Area in Saudi Arabia. N SLETTE
(I CN) 28(2): 9–15.
slam M. . . smail A. o
a . Catastrophic die-off of globally
threatened Arabian Oryx and Sand Gazelle in the fenced protected
area of the arid central Saudi Arabia. Journal of Threatened Taxa
2(2): 677–684; http://dx.doi.org/10.11609/JoTT.o2174.677-84
slam .M. . acher A. o
. rons i
. Population
development of re-introduced Mountain Gazelle in the western
Empty uarter (Uruq Bani Ma arid Protected Area), Saudi
Arabia, pp. 180–184. In: Soorae, P.S. (ed.). Global re-introduction
perspectives: 2011. Additional case studies from around the globe.
IUCN/SSC Re-introduction Specialist Group (RSG), Abu Dhabi, UAE.
ed ist
. http://www.iucnredlist.org
A
. uarter of antelope species in danger of
extinction. IUCN Red List of Threatened Species. Gnusletter 28(1):
2–5.
. IUCN/SSC Guidelines For Re-Introductions. Prepared
by the SSC Re-introduction Specialist Group. IUCN Council, Gland
Switzerland.
an l
. .
t .
rler M. ence . o aya
A. ence
. Rediscovery of a new mountain gazelle population and
clarification of taxonomic status of the genus Gazella in Turkey
using mtDNA sequencing. Folia Zoologica 61(2): 129–137.
in s ood . .
. . hairallah
. Lebanon, pp. 99–101.
In: Mallon, D.P. & S.C. Kingswood (eds.). Antelopes. Part North
Africa, the iddle East, and Asia. lobal Survey and egional Action
Plans. IUCN, Gland, Switzerland.
er
. . rons i M. Plath A. chr ter M. Pfennin er
.
Phylogenetic and population genetic analyses confirm the existence
of a distinct species boundary between Mountain (Gazella gazella)
and Arabian Gazelles (G. arabica) in the Levant. ammalian Biology
78: 220–225.
Ma in .
. Survey of gazelle species in the south-west of Saudi
Arabia. Unpublished Report, King Khalid Wildlife Research Centre,
Thumamah, Saudi Arabia.
Ma in .
. Survey of gazelle populations in south-west Saudi
Arabia and recommendations for their conservation, pp. 138–148.
In: Greth, A., C. Magin & M. Ancrenaz (eds.). Conservation of
Arabian Gazelles. National Commission for Wildlife Conservation
and Development, Riyadh, Saudi Arabia.
Ma in . . A. reth
. Distribution, status and proposals for
the conservation of Mountain Gazelles Gazella gazella cora in the
southwest of Saudi Arabia. Biological Conservation 70(1): 69–75;
http://dx.doi.org/10.1016/0006-3207(94)90300Mallon .P.
M. Al afadi
. Saudi Arabia, pp. 63–68. In:
Mallon, D.P. & S.C. Kingswood (eds.). Antelopes, Part
North
Africa, the iddle East, and Asia. IUCN Global Survey and Regional
Action Plans, Gland, Switzerland.
Mallon .P.
. . in s ood
. Antelopes. Part North Africa,
the iddle East, and Asia. lobal Survey and egional Action Plans.
IUCN, Gland, Switzerland.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
e introd ction of Arabian a elles in central a di Arabia
slam et al.
Masseti M.
. Artiodactyls of Syria. Zoology in the Middle East
33(1): 139–148; http://dx.doi.org/10.1080/09397140.2004.10638
072
ader .A.
. Rare and endangered mammals of Saudi Arabia.
In: Abu-Zinada, A.H., P.D. Goriup & I.A. Nader (eds.). Wildlife
conservation and development in Saudi Arabia. Proceedings of the
First Symposium, iayadh
. National Commission for Wildlife
Conservation and Development (NCWCD), Publication No. 3,
Riayadh.
stro s i .
. . illiams
. Heterothermy of free-living
Arabian sand gazelles ( a ella subgutturosa marica) in a desert
environment. Journal of Experimental Biology 209: 1421–1429;
http://dx.doi.org/10.1242/jeb.02151
aleh M.A.
. Chapter 7. Egypt, pp. 48–54. In: Mallon, D.P. &
S.C. Kingswood (eds.). lobal Survey and egional Action Plans
Antelopes. Part North Africa, the iddle East, and Asia. IUCN,
Gland, Switzerland.
amo r . .
. United Arab Emirates, pp. 74–78. In: Mallon, D.P.
& S.C. Kingswood (eds.). Antelopes, Part North Africa, the iddle
East, and Asia. IUCN Global Survey and Regional Action Plans,
Gland, Switzerland.
eddon P. . . an ee i
.A. ader
. Mammals of the
Harrat al-Harrah protected area, Saudi Arabia. Zoology in the
Middle East 14(1): 37–46; http://dx.doi.org/10.1080/09397140.19
97.10637702
hah M. . M. . slam A. o
. Re-introduction of Arabian
Gazelles (Gazella arabica) in fenced Protected Area in central Saudi
Arabia. IUCN Re-introduction book, 174–179pp.
halmon .
. The Arava gazelle. Israel - Land and Nature 13:
15–18.
ho less . .
. Al assri
. Taxonomic status of the Farasan
Island gazelle. Journal of Zoology (London) 223(1): 151–159; http://
dx.doi.org/10.1111/j.1469-7998.1991.tb04756.x
ho less . . . rain er M. hobra
. abibi
. Conservation
status of gazelles in Saudi Arabia. Biological Conservation 58: 85–98;
http://dx.doi.org/10.1016/0006-3207(91)90046-C
ho less . . . abibi . Ma in
. . acher
. Status and
distribution of gazelles in Saudi Arabia, pp. 52–67. In: Habibi, K.,
A.H. Abuzinada & I.A. Nader (eds.). The Gazelles of Arabia. National
Commission for Wildlife Conservation and Development, Riyadh,
Saudi Arabia.
reydte A. . . . illiams . edin . stro s i P. . eddon
.A. Marschall .A. aite
. smail
. In search of the
optimal management strategy of Arabian Oryx in Mahazat as-Sayd,
Saudi Arabia. Animal Conservation 4(3): 239–249; http://dx.doi.
org/10.1017/S1367943001001287
er mann .P.
. The ancient distribution of ungulate mammals
in the Middle East. Beiheft zum T binger Atlas des Vorderen
Orients. Reihe A (Naturwissenschaften) Nr. 27. Ludwig Reichert
Verlag. Wiesbaden 1987.
assey it erald . . . .
. Wildlife in Arabia. Oryx 1: 232–235.
acher . .
. Gazelle and camel surveys: Harrat Al Harrah and
Al Khunfah Protected Areas, Saudi Arabia. Unpublished Report, King
Khalid Wildlife Research Centre, Thumamah, Saudi Arabia.
acher . .
. Harrat Uwayrid gazelle survey. Unpublished
Report, King Khalid Wildlife Research Centre, Thumamah, Saudi
Arabia.
acher . .
. Ala eel
. Gazelle survey: the Gulf of Aqaba
between Maqna and Haqil. Unpublished Report, King Khalid Wildlife
Research Centre, Thumamah, Saudi Arabia.
acher . .
. Ala eel
a . Gazelle survey: Makshush & Al
Hayla. Unpublished Report, King Khalid Wildlife Research Centre,
Thumamah, Saudi Arabia.
acher . .
. Ala eel
b . Reconnaissance survey of Jabal
Shada. Unpublished Report, King Khalid Wildlife Research Centre,
Thumamah, Saudi Arabia.
acher . . M. Al o m
. Gazelle survey: visit to Wadi Shirs
near Tibalah. Unpublished Report, King Khalid Wildlife Research
Centre, Thumamah, Saudi Arabia.
acher . .
M. tra ss
. Field survey: Ra’s Suwaihil and
Gulf of Aqaba. Unpublished Report, King Khalid Wildlife Research
Centre, Thumamah, Saudi Arabia.
rons i . M. . slam
M. Plath
a . Latrine surveys as a
method to estimate the population size of Mountain Gazelle
(Gazella arabica). ammalian Biology 78(3): 226–230; http://
dx.doi.org/10.1016/j.mambio.2012.07.158
rons i . . Ala eel M. Plath M.A. ando a
b . Twenty
years monitoring re-introduced Mountain Gazelles in the Ibex
Reserve, Saudi Arabia. Zoology in the Middle East 55: 3–18.
rons i . M.A. ando a
.M. tyns i
c . Twenty years
conservation and monitoring of re-introduced Mountain Gazelle in
the Ibex Reserve, Saudi Arabia, pp. 175–179. In: Soorae, P.S. (ed.).
Global re-introduction perspectives: 2011. Additional case studies
from around the globe. IUCN/SSC Re-introduction Specialist Group
(RSG), Abu Dhabi, UAE.
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6053–6060
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
A
Sanjan Thapa
ISSN
Online 0974–7907
Print 0974–7893
Small Mammals Conservation and Research Foundation, New Baneshwor, Kathmandu, Nepal
sanjan smcrf.org
P
A
Abstract: A review of the literature on the mammals of Nepal revealed a series of checklists improving in accuracy over time. However,
there are contradictions in these checklists and there has been no checklist published since 1975. Here, I present a checklist based on a
review of the literature on the mammals of Nepal. The Mammals of Nepal comprise 192 species within 37 families in 12 orders.
Keywords: Checklist, families, mammals, Nepal, orders, species.
e ali Abstract g]kfnsf :tgwf/Lk|f0fLx? ;DjlGwt k|sflzt n]v/rgfx?sf] ;dLIff ubf{ ;dos|d;Fu} k|hflt;'rLx?sf] >+[vnf ;l6stflt/ ;'wf/f]Gd'v b]lvG5 . o4lk, lt
k|hflt;'rLx?df lj/f]wfef; /x]sf5g\ / ;g\ !(&% kZrft\ s'g} k|hflt;'rL k|sflzt 5}g . g]kfnsf :tgwf/Lk|f0fLx? ;DjlGwt k|sflzt n]v/rgfx?sf] ;dLIffsf] cfwf/df, oxfF d
Pp6f k|hflt;'rL k|:t't ub{5' . g]kfndf afx| pkju{, #& kl/jf/ cGtu{t !(@ k|hfltsf :tgwf/Lk|f0fLx? kfO{G5g\ .
DOI: http://dx.doi.org/10.11609/JoTT.o3511.6061-72 | ZooBank: urn:lsid:zoobank.org:pub:89208FE7-17D3-4AD0-A96C-851E7749F8DB
Editor: Giovanni Amori, Institute of Ecosystem Studies, Rome, Italy
ate of
blication 26 July 2014 (online & print)
Manuscript details: Ms # o3511 | Received 01 February 2013 | Final received 01 March 2014 | Finally accepted 20 June 2014
itation Thapa, S. (2014). A checklist of mammals of Nepal. Journal of Threatened Taxa 6(8): 6061–6072; http://dx.doi.org/10.11609/JoTT.o3511.6061-72
o yri ht © Thapa 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and
distribution by providing adequate credit to the authors and the source of publication.
Funding: Self funded.
om etin nterest The author declare no competing interests.
Author Details: : S
T
is a researcher at Small Mammals Conservation and Research Foundation in Kathmandu. His interests include taxonomy, ecology
and conservation with particular reference to small mammals. He has a master’s degree in Zoology from the Central Department of Zoology, Tribhuvan University,
Kathmandu, Nepal.
Ac no led ements I am indebted to Harrison Institute, Sevenoaks, Kent, UK for providing literature that was very helpful in preparing this checklist. I am
sincerely thankful to Dr. Gabor Csorba, Hungarian Natural History Museum, Budapest, Hungary and Dr. Hem Sagar Baral, Himalayan Nature, Kathmandu, Nepal
for critical comments on the manuscript.
6061
Checklist of Nepal mammals
Thapa
There have been a series of mammal surveys in
Nepal since the early 1820s. Studies of mammal
collections can be found in Hodgson (1832, 1834, 1835,
1836a, 1836b, 1838, 1840, 1841a, 1841b, 1841c, 1841d,
1842, 1843, 1844a, 1844b, 1845, 1847, 1858a, 1858b),
Gray (1846, 1863), Scully (1887), Hinton (1922a, 1922b),
Hinton & Fry (1923), Thomas (1924), Fry (1925), Biswas
& Khajuria (1955, 1957), Kawamichi (1968, 1971),
Greuber (1969), Frick (1969), Weigel (1969), Worth &
Shah (1969), Chesemore (1970), Agrawal & Chakraborty
(1971), Abe (1971, 1977, 1982), Martens & Niethammer
(1972), Niethammer & Martens (1975), Mitchell (1975,
1978a, 1978b, 1979, 1980), Mitchell & Punzo (1975,
1976, 1977), Mitchell & Derksen (1976), Gregori &
Petrov (1976), Marshall (1977), Ingles et al. (1980),
Johnson et al. (1980), Green (1981), Daniel & Hanz k
(1985), Oliver (1985), Bell (1986), Newton et al. (1990),
Sawada & Harada (1995), Kock (1996), Bates & Harrison
(1997), Csorba et al. (1999), Myers et al. (2000), and
Mekada et al. (2001).
Brian H. Hodgson collected 373 specimens of
70 genera and 114 species of mammals from Nepal
(Mitchell 1975). Scully (1887) described 19 species of
bats from Nepal based upon Hodgson’s and his own
collections. Hinton (1922a) distinguished Soriculus
nigrescens subspecies deposited in the British Museum
in which he described the subspecies S.n. centralis
from Nepal collected by N.A. Baptista. Hinton (1922b)
described house rats of Nepal including four subspecies
of attus rattus, . rattoides and . nitidus. Hinton & Fry
(1923) published an annotated checklist of 81 genera
and 119 species of mammals based on collections
by Lt. Colonel R.L. Kennion and N.A. Baptista from
August 1920 to March 1921. Hinton (1924) described
a new field mouse Apodemus gurkha collected by N.A.
Baptista from Laprak, Gorkha on 09 May 1923. Fry
(1925) supplemented the annotated checklist of Hinton
& Fry (1923). He described 44 species of mammals
collected by N.A. Baptista. Lindsay (1929) described
a new squirrel Sciuropterus gorkhali (now Petaurista
elegans) from Nepal on the basis of eight specimens
collected by N.A. Baptista from Gorkha listed in Hinton
& Fry (1923). Biswas & Khajuria (1955) reported
two new species Ochotona angda ai and Alticola
bhatnagari and two new subspecies attus rattus
khumbuensis and Mus musculus pygmaeus. Biswas &
Khajuria (1957) described a collection of 52 specimens
of 21 species and subspecies. They reported the first
record of Beech Marten Martes foina intermedia from
6062
Nepal. Frick (1969) produced a checklist of 169 species
and subspecies of mammals found in Nepal. Caughley
(1969) listed 16 genera and 17 species of mammals from
the Trishuli watershed. A German research expedition
in 1961–62 visited the Khumbu region of the Nepalese
Himalayas and collected 314 skins and skulls of
insectivores and rodents. Greuber (1969) explained the
occurrence of species in relation to biotope and altitude.
Wiegel (1969) produced an annotated checklist of the
species, discussed insectivores and rodents, reported
a new species Soriculus gruberi and a new subspecies
Sorex cylindricauda nipalensis, demonstrated Mus
musculus pygmaeus reported previously by Biswas &
Khajuria (1955) is a young of M.m. homourus and the
species were discussed in relation to distribution in the
zoo-geographic regions. Worth & Shah (1969) collected
specimens of mammals from Nepal for ectoparasites
studies. It included 27 specimens of five genera and
three families of bats which were collected from
Kathmandu, Pokhara and eastern Tarai by R.M. Mitchell
(Mitchell 1978a). Chesemore (1970) noted 40 species
of mammals mainly from southern Nepal. Agrawal &
Chakraborty (1971) examined the collection of small
mammals by R.M. Mitchell from Nepal. They published
a note describing a new species Ochotona mitchelli.
Abe (1971, 1977) described taxonomic and ecological
data for 570 small terrestrial mammals comprising 28
species collected from 33 localities in central Nepal.
Abe (1982) detailed the ecological distribution and
the faunal structure of central Nepal’s small ground
mammal fauna.
Martens & Niethammer (1972)
recorded a new species Apodemus sylvaticus ardi
(currently considered a synonym of Apodemus pallipes)
for Nepal and collected new material of A. gurkha.
Also, they mentioned the distribution pattern of both
species. Niethammer & Martens (1975) discussed the
genera attus and Maxomys (now Niviventer) from
Afghanistan and Nepal based upon the specimens
collected by Martens from Nepal. Mitchell (1975)
prepared a checklist of 145 species and subspecies of
mammals (excluding bats) based upon 4,000 terrestrial
mammal specimens representing 130 species collected
by the Nepal Ectoparasite Program between 1967
and 1970. Mitchell & Punzo (1975) described a new
species Ochotona lama (now O. nubrica) from Nepal.
Mitchell & Punzo (1976) discussed five new records of
mammals from Nepal namely, Ovis ammon hodgsoni,
Tragulus meminna, Crocidura attenuata, Suncus
stoliczkanus and S. estruscus pygmaeoides. Mitchell
& Derksen (1976) reported mammals of nine species
of four orders. Mitchell (1978a) prepared a checklist
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Checklist of Nepal mammals
of 18 genera and 37 species of bats among which 15
genera and 17 species were collected from Nepal Health
Survey, Nepal Ectoparasite Program and Arun Valley
Wildlife Expedition. Six new records were listed in the
checklist. Mitchell (1978b) described six species of pikas
from Nepal based upon 155 specimens collected by the
Nepal Ectoparasite Program. Mitchell (1979) provided
accounts of 11 species of eight genera of sciurid rodents
from Nepal. Mitchell (1980) reported new records of
five species from Nepal.
Gregori & Petrov (1976) described six species based
upon 46 specimens from Makalu-Barun collected
by J. Gregori during the 1972 Yugoslav Himalayan
Expedition. In 1977, Joe T. Marshall, Jr. published an
erudite monograph on Asian species of the genus Mus,
which included the collection locality of specimens of
Mus cervicolor, M. cookii, and M. musculus collected
by the author and Stephen C. Frantz from Tiger Tops in
Royal Chitwan National Park, Hetauda, and Kathmandu.
Jhonson et al.(1980) described 35 species of mammals
with three new species namely, Tupaia glis (now T.
belangeri), Vulpes bengalensis and Lepus grahami (now
L. oiostolus) based upon 112 specimens collected by S.D.
Ripley from 1948–1949. Ingles et al. (1980) reported
the first record of Diomys crumpi and records of other
three shrews based upon the specimens deposited in
the British Museum of Natural History collected by the
University of East Anglia Expedition to Nepal from 1978–
1979.
Green (1981) published a checklist with notes on
some mammals from Langtang National Park. Daniel
& Hanz k (1985) examined taxonomic and ecological
aspects of 139 specimens of six species of mammals
from Makalu-Barun, which was collected by the
Czechoslovakian Expedition from 26th March and 25th
May, 1973. Oliver (1985) surveyed Chitwan National
Park, Bardia National Park, and Sukla Phanta Wildlife
Reserve and reported the presence of Caprolagus
hispidus in all three protected areas, but evidence
of Porcula salvania was not confirmed. Bell (1986)
confirmed an occurrence of four male and three female
C. hispidus at two sites in the Sukla Phanta Wildlife
Reserve together with two Indian hares (Lepus nigricollis
ru caudatus) at the Reserve’s camp at Pipariya. Newton
et al. (1990) described the collection of 71 specimens
of 11 species of Muridae and Soricidae from nine
localities in Nepal collected by the University of East
Anglia Expedition to Nepal from 1978–1979. Suwal &
Verheugt (1995) enumerated a checklist of 181 species
of 39 families of 12 orders of mammals. Kock (1996)
discussed a collection of 10 species of chiroptera from
Thapa
Nepal (Nine species were collected by Jochen Martens,
of the University of Mainz, between December, 1969
and May, 1973 and one specimen of Pteropus giganteus
presented by B.H. Hodgson). Bates & Harrison (1997)
compiled detailed information on 49 bat species from
Nepal. Information was gathered through museum
visits, literature study and short field seasons, in which
specimens of six species were collected and deposited
in the Harrison Zoological Museum. Topal (1997)
explained the yotis longipes collected from Nepal to
be different from collections at other locations. Csorba
et al. (1999) reported recent records of chiroptera from
Nepal, with remarks on their natural history based
upon 23 bat species collected by Russian and Hungarian
expeditions. They added first records of three species
namely Ia io, urina cyclotis, and Kerivoula hard ickii
from Nepal. They also prepared a checklist of 51 species
known as of that date from Nepal in an appendix.
They focused on yotis csorbai, which proved to be
a new species. Myers et al. (2000) lately summarized
their field work in and near Chitwan National Park
during March 1990 based upon the collection of 143
specimens of 14 bat species. They reported first records
of Eonycteris spelaea and Eptesicus dimissus as well as
verified presence of Miniopterus pusillus and Kerivoula
picta. Mekada et al. (2001) conducted a faunal survey
and collected 131 specimens of insectivores and rodents
from the Annapurna region and outskirts of Kathmandu
City in 1996 and 1999. Majupuria & Kumar Majupuria
(2006) published a book with a checklist of 187 species
of mammals reported from Nepal including Yeti. Baral
& Shah (2008) presented a checklist of 208 species of
mammals including humans in the book Wild Mammals
of Nepal .
Checklists on Nepalese mammals have been
periodically refined since Hinton & Fry (1923). Fry
(1925), Frick (1969), Mitchell (1975), Mitchell (1978a),
Csorba et al. (1999), Yonzon (2004), and Majupuria &
Kumar Majupuria (2006) were the primary publications
of checklists of mammals. A checklist of mammals found
in Nepal in Baral & Shah (2008) is the latest. However,
there are contradictions in these checklists. There has
been no attempt in updating a standard checklist on
mammals from Nepal in a journal since Mitchell (1975).
Thus I aim to fill this gap in knowledge.
MA
A
A
M
I prepared a checklist based on a review of
literature regarding mammals of Nepal. Abe (1971,
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
6063
Checklist of Nepal mammals
Thapa
1977), Mitchell (1975), Johnson et al. (1980), Molur
et al. (2002, 2005), Baral & Shah (2008), Acharya et al.
(2010), Pearch (2011), Jnawali et al. (2011), and Thapa
et al. (2012) were the major source of information on
species. Similarly, Suwal & Verheugt (1995), Shrestha
(1997) and Majupuria & Kumar Majupuria (2006) were
additionally cited. Taxonomic updates are based on
Wilson & Reeder (2005) and IUCN (2013).
Careful analysis and verification of the presence
and absence of mammals confirmed occurrence of
192 species of mammals representing 37 families and
12 orders. It includes two endemic species; Apodemus
gurkha and yotis csorbai. Two species new to Nepal
are added in this checklist namely; Mus pahari and
Scotozous dormeri. A checklist of mammal species that
are confirmed to occur in Nepal is given in Table 1.
Thomas & Hinton (1922) described the 52 specimens
of 10 species deposited in the British Museum collected
by A.F.R. Wollaston during the 1921 Mount Everest
Expedition. All the collection localities fall in Tibet.
Unfortunately, the species described in this paper were
added to the Nepalese checklist. Therefore, some
species have not yet been discovered from the country
or they do not occur here (Table 2).
Pearch (2011) clearly updated the small mammals
of Nepal enumerating 118 species; however, I disagree
with some species inclusions and exclusions in his list
until and unless a satisfactory field assessment of the
fauna is undertaken. Therefore, with respect to Pearch
(2011), I include Eptesicus gobiensis as a probable
species of a small mammal of Nepal. Sorex thibetanus is
included in Pearch (2011). However, this species is still
subject to taxonomic controversy, with little conclusive
information currently available for the species (Hutterer
2005) and it is considered endemic to China (Smith & ie
2008). Agrawal & Chakraborty (1971) labeled a specimen
Sorex araneus from Nepal. Hoffmann (1987) questioned
the identity of the specimen, however, suggesting that
it may be assignable to S. excelsus (Chakraborty et al.
2004; Pearch 2011). S. araneus is restricted to Europe
according to IUCN (2013). Therefore, I include S.
excelsus in this list for the specimen collected by Agrawal
& Chakraborty (1971).
6064
I doubt the presence of Sphaerias blanfordi in the
country as Lekagul & McNeely (1977) mentioned no
specific location other than eastern Nepal without any
further details (Bates & Harrison 1997; Pearch 2011).
Similarly, the taxonomy of Rhinolophus subbadius
is controversial as the holotype of the species from
Nepal could not be traced (Csorba et al. 2003; Pearch
2011). The locality for yotis siligorensis listed as
Siligori, Nepal is erroneous as Siligori is in India. This
brings into question the occurrence of the species in
Nepal. On this point, I agree with Pearch (2011), but
the unconfirmed identification of a yotis mystacinus
siligorensis specimen in the collections of the Museum
of Comparative Zoology, Harvard University (MCZ
32977) hints further taxonomic research is required.
Therefore, I keep M. siligorensis as a probable species
for Nepal. yotis mystacinus is confined to Europe and
the specimens referred as M. mystacinus have been
reassessed as M. nipalensis by Benda and Tystsulina
(2000), therefore, I exclude M. mystacinus from the list
of Pearch (2011).
With respect to Jnawali et al. 2011, I include Mus
platythrix as a probable species of small mammal
from Nepal. Ochotona himalayana is speculated to be
distributed in Nepal; there is no record of this species.
Hence, I do not include this species in the checklist.
Ochotona thibetana perhaps can be found in SheyPhoksundo National Park (SPNP) (Suwal & Verheugt
1995; Shrestha 1997; Majupuria & Kumar Majupuria
2006). However, Smith & Boyer (2008) show its
distribution in high mountains near the Tibetan border
in eastern Nepal (Thapa et al. 2011). Because there is no
record of specimens of this species from Nepal I exclude
it from the list. Semnopithecus ajax is restricted to India,
however, Brandon-Jones (2004) claimed its occurrence
from Nepal on the basis of a skin from Melamchi (Groves
& Molur 2008). Hence, further confirming studies are
necessary.
Corbet & Hill (1992) mapped the distribution of
Scotophilus kuhlii from the Tarai region of Nepal, but,
there had been no record of a voucher specimen. Two
specimens of S. kuhlii (CDZ TU BAT 030; CDZ TU BAT
032) are deposited in the Museum of Central Department
of Zoology, Tribhuvan University, Kathmandu, Nepal.
Recently, Thapa et al. (2012) recorded Scotozous
dormeri to Nepal.
The presence of Indian Chevrotain Moschiola
indica is uncertain from Nepal (IUCN 2013), Baral et al.
(2009) raised the need of additional survey. The Pygmy
Hog Porcula salvania, Wild Yak Bos mutus and Chiru
Pantholops hodgsonii appear to be extinct from Nepal
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Checklist of Nepal mammals
Thapa
able . hec list of mammals of e al
Species
ommon name
Species
ommon name
Order Proboscidea
28. Bandicota bengalensis (Gray, 1835)
Lesser Bandicoot Rat
Family Elephantidae
29. Bandicota indica (Bechstein, 1800)
Greater Bandicoot
Rat
30. Dacnomys millardi Thomas, 1916
Millard’s Rat
31. Diomys crumpi Thomas, 1917
Crump’s Mouse
32. olunda ellioti Gray, 1837
Indian Bush Rat
33. Millardia meltada (Gray, 1837)
Soft-furred Metad
34. Mus booduga (Gray, 1837)
Common Indian Field
Mouse
1. Elephas maximus Linnaeus, 1758
Asian Elephant
Order Scandentia
Family Tupaiidae
2. Tupaia belangeri (Wagner, 1841)
NorthernTree-Shrew
Order Primates
Family Cercopithecidae
3. Macaca assamensis (M Clelland, 1840)
4.
acaca mulatta (Zimmermann, 1780)
Assam Macaque
Rhesus Monkey
5. Semnopithecus ajax (Pocock, 1928)
Himalayan Gray
Langur
6. Semnopithecus hector (Pocock, 1928)
Tarai Gray Langur
7. Semnopithecus schistaceus Hodgson, 1840
Nepal Gray Langur
Family Hominidae
8. Homo sapiens Linnaeus, 1758
Human
Order Rodentia
Family Sciuridae
35. Mus cervicolor Hodgson, 1845
Fawn-colored Mouse
36. Mus cookii Ryley, 1914
Cook’s Mouse
37. Mus musculus Linnaeus, 1758
House Mouse
38. Mus pahari Thomas, 1916
Sikkim Mouse
39. Mus phillipsi Wroughton, 1912
Wroughton’s Small
Spiny Mouse
40. Mus saxicola Elliot, 1839
Brown Spiny Mouse
41. Mus terricolor Blyth, 1851
Earth-colored Mouse
42. Nesokia indica (Gray, 1830)
Short-tailed
Bandicoot Rat
43. Niviventer eha (Wroughton, 1916)
Little Himalayan Rat
9. Belomys pearsonii (Gray, 1842)
Hairy-footed Flying
Squirrel
44. Niviventer fulvescens (Gray, 1847)
Chestnut Whitebellied Rat
10. Callosciurus pygerythrus (I. Geoffroy Saint
Hilaire, 1832)
Hoary-bellied
Squirrel
45. Niviventer niviventer (Hodgson, 1836)
Himalayan Whitebellied Rat
11. Dremomys lokriah (Hodgson, 1836)
Orange-bellied
Himalayan Squirrel
46. attus andamanensis (Blyth, 1860)
Indochinese Forest
Rat
12. Hylopetes alboniger (Hodgson, 1836)
Particolored Flying
Squirrel
47. attus nitidus (Hodgson, 1845)
Himalayan Field Rat
48. attus pyctoris (Hodgson, 1845)
Himalayan Rat
49. attus rattus (Linnaeus, 1758)
House Rat
50. attus tane umi Temminck, 1844
Oriental House Rat
13. Funambulus pennanti Wroughton, 1905
Five-striped Palm
Squirrel
14. Marmota himalayana (Hodgson, 1841)
Himalayan Marmot
15. Petaurista elegans (M ller, 1840)
Spotted Giant Flying
Squirrel
51. Tatera indica (Hardwicke, 1807)
Indian Gerbil
16. Petaurista magni cus (Hodgson, 1836)
Hodgson’s Giant
Flying Squirrel
52. Vandeleuria oleracea (Bennett, 1832)
Asiatic Long-tailed
Climbing Mouse
17. Petaurista nobilis (Gray, 1842)
Bhutan Giant Flying
Squirrel
Family Hystricidae
18. Petaurista petaurista (Pallas, 1766)
Red Giant Flying
Squirrel
19. Ratufa bicolor (Sparrman, 1778)
Black Giant Squirrel
20. Tamiops macclellandi (Horsefield, 1840)
Himalayan Striped
Squirrel
Family Spalacidae
21. Cannomys badius (Hodgson, 1841)
Lesser Bamboo Rat
Family Cricetidae
22. Alticola stolic kanus (Blanford, 1875)
Stoliczka’s Mountain
Vole
23. Cricetulus alticola Thomas, 1917
Tibetan Dwarf
Hamster
24. Neodon sikimensis (Horsefield, 1841)
Sikkim Vole
25. Phaiomys leucurus Blyth, 1863
Blyth’s Vole
Family Muridae
26. Apodemus gurkha Thomas, 1924
Himalayan Wood
Mouse
27. Apodemus pallipes (Barrett-Hamilton, 1900)
Himalayan Field
Mouse
53. Hystrix brachyura Linnaeus, 1758
Malayan Porcupine
54. Hystrix indica Kerr, 1792
Indian Crested
Porcupine
Order Lagomorpha
Family Ochotonidae
55. Ochotona curzoniae (Hodgson, 1858)
Black-lipped Pika
56. Ochotona macrotis (G nther, 1875)
Large-eared Pika
57. Ochotona nubrica Thomas, 1922
Nubra Pika
58. Ochotona roylei (Ogilby, 1839)
Royle’s Pika
Family Leporidae
59. Caprolagus hispidus (Pearson, 1839)
Hispid Hare
60. Lepus nigricollis F. Cuvier, 1823
Indian Hare
61. Lepus oiostolus Hodgson, 1840
Woolly Hare
Order Eulipotyphla
Family Soricidae
62. Chimarrogale himalayica (Gray, 1842)
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Himalayan Water
Shrew
6065
Checklist of Nepal mammals
Species
Thapa
ommon name
Species
ommon name
63. Crocidura attenuata Milne-Edwards, 1872
Indo-Chinese Shrew
Family Vespertilionidae
64. Episoriculus caudatus (Horsefield, 1851)
Hodgson’s Browntoothed Shrew
94. Arieluluscircumdatus (Temminck, 1840)
Bronze Sprite
65. Episoriculus leucops (Horsefield, 1855)
Long-tailed Browntoothed Shrew
95. Barbastella leucomelas (Cretzschmar, 1826)
Eastern Barbastelle
Surat Serotine
66. Episoriculus macrurus (Blanford, 1888)
Long-tailed
Mountain Shrew
96. Eptesicus dimissus Thomas, 1916
97. Eptesicus serotinus (Schreber, 1774)
Serotine
Chocolate Pipistrelle
67. Nectogale elegans Milne-Edwards, 1870
Elegant Water Shrew
98. Falsistrellu sa nis (Dobson, 1871)
68. Sorex bedfordiae Thomas, 1911
Lesser Stripe-backed
Shrew
99. Hesperoptenus tickelli (Blyth, 1851)
Tickell s Bat
100. Ia io Thomas, 1902
Great Evening Bat
101. Kerivoula hard ickii (Horsfield, 1824)
Hardwicke s Woolly
Bat
102. Kerivoula picta (Pallas, 1767)
Painted Woolly Bat
103. Murina aurata Milne-Edwards, 1872
Tibetan Tube-nosed
Bat
104.
urina cyclotis Dobson, 1872
Round-eared Tubenosed Bat
105.
urina huttoni (Peters, 1872)
White-bellied Tubenosed Bat
69. Sorex excelsus G.M. Allen, 1923
Highland Shrew
70. Sorex minutus Linnaeus, 1766
Eurasian Pygmy
Shrew
71. Soriculus nigrescens (Gray, 1842)
Himalayan Shrew
72. Suncus estruscus (Savi, 1822)
Pygmy Whitetoothed Shrew
73. Suncus murinus (Linnaeus, 1766)
Asian House Shrew
74. Suncus stoliczkanus (Anderson, 1877)
Anderson’s Shrew
Family Talpidae
75. Euroscaptor micrura (Hodgson, 1841)
Himalayan Mole
106. Murina leucogaster Milne-Edwards, 1872
Greater Tube-nosed
Bat
Order Chiroptera
107.
yotis blythii (Tomes, 1857)
Lesser Mouse-eared
Myotis
Family Pteropodidae
108.
yotis csorbai Top l, 1997
Csorba’s Mouseeared Myotis
76. Cynopterus sphinx (Vahl, 1797)
Greater Short-nosed
Fruit Bat
109.
yotis formosus (Hodgson, 1835)
Hodgson s Bat
77. Eonycteris spelaea (Dobson, 1871)
Dawn Bat
110.
yotis muricola (Gray, 1864)
Nepalese Whiskered
Myotis
111.
yotis nipalensis Dobson, 1871
Nepal Myotis
112.
yotis sicarius Thomas, 1915
Mandelli s Mouseeared Myotis
78. Pteropus giganteus (Br nnich, 1782)
Indian Flying Fox
79. ousettus leschenaulti (Desmarest, 1820)
Leschenault’s
Rousette
Family Rhinolophidae
113. Nyctalus montanus (Barrett-Hamilton, 1906)
Mountain Noctule
114. Nyctalus noctula (Schreber, 1774)
Noctule
Greater Horseshoe
Bat
115. Philetor brachypterus (Temminck, 1840)
Short-winged
Pipistrelle
82. Rhinolophus luctus Temminck, 1834
Great Woolly
Horseshoe Bat
116. Pipistrellus coromandra (Gray, 1838)
Coromandel
Pipistrelle
83. Rhinolophus lepidus Blyth, 1844
Blyth’s Horseshoe
Bat
117. Pipistrellus javanicus (Gray, 1838)
Javan Pipistrelle
118. Pipistrellus tenuis (Temminck, 1840)
Least Pipistrelle
80. hinolophus a nis Horsfield, 1823
Intermediate
Horseshoe Bat
81. Rhinolophus ferrumequinum (Schreber, 1774)
84. hinolophus macrotis Blyth, 1844
Big-eared Horseshoe
Bat
Pearson’s Horseshoe
Bat
119. Plecotus auritus (Linnaeus, 1758)
Brown Big-eared Bat
85. Rhinolophus pearsonii Horsfield, 1851
120. Plecotus austriacus (J. Fischer, 1829)
Gray Big-eared Bat
86. Rhinolophus pusillus Temminck, 1834
Least Horseshoe Bat
121. Scotomanes ornatus (Blyth, 1851)
Harlequin Bat
87. Rhinolophus sinicus K. Andersen, 1905
Chinese Horseshoe
Bat
122. Scotophilus heathii (Horsfield, 1831)
Greater Asiatic
Yellow HouseBat
123. Scotophilus kuhlii Leach, 1821
Lesser Asiatic Yellow
House Bat
Dormer’s Bat
Family Hipposideridae
88. Hipposideros armiger (Hodgson, 1835)
Great Himalayan
Leaf-nosed Bat
124. Scotozous dormeri Dobson, 1875
89. Hipposideros cineraceus Blyth, 1853
Least Leaf-nosed Bat
Family Miniopteridae
90. Hipposideros fulvus Gray, 1838
Fulvus Leaf-nosed
Bat
125. Miniopterus fuliginosus (Hodgson, 1835)
Eastern Bent-winged
Bat
91. Hipposideros pomona K. Andersen, 1918
Andersen s Leafnosed Bat
126. Miniopterus pusillus Dobson, 1876
Small Long-fingered
Bat
Order Pholidota
Family Megadermatidae
92. Megaderma lyra . Geoffroy, 1810
Greater False
Vampire
Family Emballonuridae
93. Taphozous longimanus Hardwicke, 1825
6066
Long-winged Tomb
Bat
Family Manidae
127. Manis crassicaudata E.Geoffroy, 1803
Indian Pangolin
128. Manis pentadactyla Linnaeus, 1758
Chinese Pangolin
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Checklist of Nepal mammals
Species
Thapa
ommon name
Species
ommon name
Order Carnivora
163. Mellivora capensis (Schreber, 1776)
Family Felidae
164. Mustela altaica Pallas, 1811
Mountain Weasel
165. Mustela kathiah Hodgson, 1835
Yellow-bellied
Weasel
166. Mustela sibrica Pallas, 1773
Siberian Weasel
167. Mustela strigidorsa Gray, 1853
Stripe-backed
Weasel
129. Felis chaus Schreber, 1777
Jungle Cat
130. Lynx lynx (Linnaeus, 1758)
Eurasian lynx
131. Neofelis nebulosa (Gri th, 1821)
Clouded Leopard
132. Panthera pardus (Linnaeus, 1758)
Leopard
133. Panthera tigris (Linnaeus, 1758)
Royal Bengal Tiger
Family Ailuridae
134. Panthera uncia (Schreber, 1775)
Snow Leopard
168. Ailurus fulgens F.G. Cuvier, 1825
135. Pardofelis marmorata (Martin, 1837)
Marbled Cat
Order Perissodactyla
136. Pardofelis temminckii (Vigors & Horsefield,
1827)
Asiatic Golden Cat
137. Prionailurus bengalensis (Kerr, 1792)
Leopard Cat
138. Prionailurus viverrinus (Bennett, 1883)
Fishing Cat
Family Viverridae
169. Equus kiang Moorcroft, 1841
170. Rhinoceros unicornis Linnaeus, 1758
Order Cetartiodactyla
140. Paguma larvata (C.E.H. Smith, 1827)
Masked Palm Civet
Family Suidae
141. Paradoxurus hermaphroditus (Pallas, 1777)
Common Palm Civet
171. Sus scrofa Linnaeus, 1758
142. Prionodon pardicolor Hodgson, 1842
Spotted Linsang
Family Tragulidae
143. Vivericula indica ( . Geoffroy Saint-Hilaire,
1803)
Small Indian Civet
144. Viverra zibetha Linnaeus, 1758
Large Indian Civet
Indian Grey
Mongoose
146. Herpestes javanicus ( .Geoffroy Saint-Hilaire,
1818)
Small Asian
Mongoose
147. Herpestes urva (Hodgson, 1836)
Crab-eating
Mongoose
Family Hyaenidae
148. Hyaena hyaena (Linnaeus, 1758)
Striped Hyaena
Family Canidae
149. Canis aureus Linnaeus, 1758
Golden Jackal
150. Canis lupus Linnaeus, 1758
Gray Wolf
151. Cuon alpinus (Pallas, 1811)
Dhole
152. Vulpes bengalensis (Shaw, 1800)
Bengal Fox
153. Vulpes ferrilata Hodgson, 1842
Tibetan Fox
154. Vulpes vulpes (Linnaeus, 1758)
Red Fox
Family Ursidae
157. Melursus ursinus (Shaw, 1791)
Sloth bear
155. Ursus arctos Linnaeus, 1758
Brown Bear
156. Ursus thibetanus G. Baron Cuvier, 1823
Himalayan Black
Bear
Family Mustelidae
172. Moschiola indica (Gray, 1852)
174. Moschus fuscus Li, 1981
Black Musk Deer
175. Moschus leucogaster Hodgson, 1839
Himalayan Musk
Deer
Family Cervidae
176. Axis axis (Erxleben, 1777)
Chital
177. Hyelaphus porcinus (Zimmermann, 1780)
Indian Hog Deer
178.
untiacus vaginalis (Zimmermann, 1780)
Barking Deer
179. Rucervus duvaucelii (G. Cuvier, 1823)
Barasingha
180. Rusa unicolor (Kerr, 1792)
Sambar Deer
Family Bovidae
181. Antilope cervicapra (Linnaeus, 1758)
Black Buck
182. Bubalus arnee (Kerr, 1792)
Asian Water Buffalo
183. Bos gaurus C.H. Smith, 1827
Gaur
184. Boselaphus tragocamelus (Pallas, 1766)
Nilgai
185. Capricornis thar (Hodgson, 1831)
Himalayan Serow
186. Hemitragus jemlahicus (C.H. Smith, 1826)
Himalayan Tahr
187. Naemorhedus goral (Hardwicke, 1825)
Himalayan Goral
188. Ovis ammon (Linnaeus, 1758)
Argali
189. Procapra picticaudata Hodgson, 1846
Tibetan Gazelle
190. Pseudois nayaur (Hodgson, 1833)
Blue Sheep
191. Tetracerus quadricornis (deBlainville, 1816)
Chousingha
159. Lutra lutra (Linnaeus, 1758)
Eurasian Otter
Order Cetacea
Smooth-coated Otter
Family Platinistidae
162. Martes foina (Erxleben, 1777)
Indian Chevrotain
Alpine Musk Deer
160. Lutrogale perspicillata (I. Geoffroy SaintHilaire, 1826)
Yellow-throated
Marten
Wild Boar
173. Moschus chrysogaster (Hodgson, 1839)
Oriental Smallclawed Otter
artes flavigula (Boddaert, 1758)
Greater One-horned
Rhino
Family Moschidae
158. Aonyx cinerea (Illiger, 1815)
161.
Kiang
Family Rhinocerotidae
Binturong
145. Herpestes ed ardsii ( . GeoffroySaint-Hilaire,
1818)
Red Panda
Family Equidae
139. Arctictis binturong (Ra es,1821)
Family Herpestidae
Honey Badger
192. Platanista gangetica gangetica (Roxburgh,
1801)
Ganges River Dolphin
Stone Marten
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
6067
Checklist of Nepal mammals
Thapa
Table 2. Species excluded in the current checklist.
Species
ited re io sly
eferences s
the decision
eason for not incl din
ortin
Ochotona lama
Jnawali et al. (2011)
Taxonomic issues, not recognized as a full species, considered sympatric to
O. nubrica
Thapa et al. (2011)
Apodemus sylvaticus
Jnawali et al. (2011)
Extralimital or hypothetical for Nepal
Schlitter et al. (2008)
Bandicota maxima
Jnawali et al. (2011)
The taxon has not been assessed for IUCN Red List
IUCN (2013)
Crocidura horse eldi
Jnawali et al. (2011)
The taxon has not been assessed for IUCN Red List
IUCN (2013)
Eptesicus gobiensis
Pearch (2011)
In absence of specimens of E. nilssoni collected by R.M. Mitchell
Sorex thibetanus
Pearch (2011)
This species is still subject to taxonomic controversy, with little conclusive
information currently available for the species (Hutterer 2005) and
considered endemic to China (Smith & ie 2008)
Sphaerias blanfordi
Acharya et al. (2010)
Lekagul & Mcneely (1977) mentions no specific location rather than eastern
Nepal without any further details
Bates & Harrison (1997),
Pearch (2011)
Rhinolophus subbadius
Acharya et al. (2010)
Holotype of the species from Nepal could not be traced
Csorba et al. (2003),
Pearch (2011)
yotis siligorensis
Acharya et al. (2010)
The locality mentioned is Siligori, Nepal is erroneous as Siligori is in India
Pearch (2011)
yotis mystacinus
Pearch (2011)
The speies is confined to Europe
Benda & Tystsulina (2000)
Alticola roylei
Jnawali et al. (2011)
Endemic to India
Molur & Nameer (2008a)
Mus platythrix
Jnawali et al. (2011)
Endemic to India
Molur & Nameer (2008c)
Crocidura pergrisea
Jnawali et al. (2011)
Endemic to Pakistan
Molur & Nameer (2008b)
Arctonyx collaris
Jnawali et al. (2011)
Distributed in to South East Asia, China, Mongolia, Bhutan and northeastern
India
Timmins et al. (2008)
Melogale personata
Jnawali et al. (2011)
there have been no subsequent records since Hodgson 1836- holotype for
subspecies
Duckworth et al. (2008)
Marmota bobak
Jnawali et al. (2011)
No specimen records and no confirming specific locality records
Jnawali et al. (2011)
- Range of its distribution out of Nepal.
- The only specimen made over by Hodgson to the British Museum is a
furrier’s skin, said to have been brought from Tibet (Hinton & Fry 1923).
- Not included in the checklist by Mitchell (1975).
Since, there has been no sighting of the animal during winter in white coat in
Nepal further research is necessary.
Mustela erminea
(Harris & Leslie 2008; Mallon 2008; Narayan et al. 2008).
Previous checklists (Baral & Shah 2008; Jnawali et
al. 2011) include 208 species. However, this checklist
reduces the number to 192 on the basis of concrete
evidence of occurrence of species through valid
specimens and literature on specific records. The
research aspect on small mammals and small cats of this
country is poor which will be fostered in coming days.
Many more new species of this category of mammals
will be added to the current checklist.
Abe .
. Small Mammals of Central Nepal - Mammalia. Journal
Faculty of Agriculture, Hokkaido niversity, Sapporo, Japan 56:
396–403.
Abe .
.Variation and taxonomy of some small mammals from
Central Nepal. Journal of the Mammalian Society of Japan 7(2):
63–73.
Abe .
. Ecological distributions of small mammals in central
Nepal. Mammalia 46: 477–503.
Acharya P. . Adhi ari . ahal A. ha a
. ha a
. Bats of
Nepal - A Field Guide. Small Mammals Conservation and Research
6068
Reid & Helgen (2008),
Blanford (1891)
Foundation, New Baneshwor, Kathmandu, 114pp.
A ra al . .
. ha raborty
. Notes on a collection of Small
Mammals from Nepal, with a description of a new mouse-hare
(Lagomorpha: Ochotonidae). Proceedings of Zoological Society,
Calcutta 24(1): 41–46.
aral . .
. . hah
. Wild Mammals of Nepal. Himalayan
Nature, Kathmandu, 158pp.
aral . . . . hah
. . c orth
. A clarification of the
status of Indian Chevrotain Moschiola indica in Nepal. Vertebrate
Zoology 59(2): 197–200.
ates P. . .
. . arrison
. Bats of Indian Subcontinent.
Harrison Zoological Museum Publication, London, 258pp.
ell . .
. A study of the Hispid Hare Caprolagus hispidusin
Royal Suklaphanta Wildlife Reserve, western Nepal: a summary
report. Dodo 23: 24–31.
is as .
. ha ria
. Zoological Results of the Daily Mail’
Himalayan Expedition, 1954, four new mammals from Khumbu,
Eastern Nepal. Proceedings of the Zoological Societyof Calcutta 8:
26–29.
is as .
. ha ria
. Zoological results of the Daily Mail’
Himalayan Expedition 1954. Notes on some mammals of Khumbu,
Eastern Nepal. Proceedings of the ZoologicalSociety of Calcutta,
ukher ee emorial olume Calcutta 229–253.
Blyth, E. (1844). Notices of various mammalia. Journal of the Asiatic
Society of Bengal 13: 463–494.
Brandon-Jones, D. (2004). A taxonomic revision of the langurs and leaf
monkeys (Primates: Colobinae) of South Asia. Zoos’ Print Journal
19(8): 1552–1594; http://dx.doi.org/10.11609/JoTT.ZPJ.971.155294
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Checklist of Nepal mammals
ha raborty . . rini as l
. rini as l M. . Pradhan P. .
ameer
. Checklist of insectivores (Mammalia: Insectivora)
of South Asia. Zoos’ Print Journal 19(2): 1361–1371; http://dx.doi.
org/10.11609/JoTT.ZPJ.19.2.1361-71
hesemore . .
. Notes on the mammals of southern Nepal.
Journal of Mammology 51: 162–166.
orbet . .
. . ill
. The Mammals of the Indomalayan
Region. Natural History Museum/OUP, 488pp.
sorba . P. heli
. homas
. Horseshoe Bats of the World
(Chioptera: Rhinolophidae). Alana Books, 160pp.
sorba . . . r s o
A. . orissen o
. Recent records of
bats (Chiroptera) from Nepal, with remarks on their natural history.
Mammalia 63(1): 61–78.
aniel M. . an
. Small mammals in eastern part of Nepal
Himalaya. Rozpravy Ceskoslovenské Akademie Ved 95(8): 1–59.
c orth . . . . immins . on P. on on . oberton
.
Q. Phuong (2008). Melogale personata. In: IUCN 2013. IUCN Red
List of Threatened Species. Version 2013.1. www.iucnredlist.org .
Downloaded on 09 July 2013.
ric
.
. Die Hohenstufenverteilung der nepalischen
Saugetiere. Saugetierkdl. itt 17(2): 161–173.
ry . .
. Report No. 37a: Nepal. Bombay Natural History
Society’s Mammal Survey of India, Burma, and Ceylon. Journal
Bombay Natural History Society 30: 525–530.
ray . .
. Catalogue of the specimens and drawings of
mammalia and birds of Nepal and Tibetpresented by B.H. Hodgson,
Esq., to the British Museum. London,156pp.
ray . .
. Catalogue of the specimens and drawings of
mammals, birds, reptiles and fishes of Nepal and Thibet presented
by B.H. Hodgson to the British Museum. Neuman, London, 156pp.
reen M. . .
. A check-list and some notes concerning the
mammals of the Langtang National Park, Nepal. Journal of the
Bombay Natural History Society 78(1): 77–87.
re ori .
. Petro
. Scientific results of the Yugoslav 1972
Himalaya expedition. Mammalia. Razprave. Slovenska Akademija
Znanosti in Umetnosti. Classis IV: Historia naturalis et medicina
19(1): 1–20.
re ber . .
. Tiergeographische, okologische and bionomische
Untersuchungen an kleinen S ugetieren in Ost-Nepal. In: Hellmich,
W. (ed.). Khumbu Himal. Ergebnisse des Forschungsunternehmens
Nepal Himalaya. Universit tsverlag Wagner Ges. M. B. H., InnsbruckM nchen3(2): 149–312. English summary .
ro es .P.
. Mol r
. Semnopithecus ajax. The IUCN Red
List of Threatened Species. Version 2014.1. www.iucnredlist.org .
Downloaded on 15 July 2014.
arris . .
. eslie
. Bos mutus. In: IUCN 2013. IUCN Red
List of Threatened Species. Version 2013.1. www.iucnredlist.org .
Downloaded on 09 July 2013.
inton M.A. .
a . Scientific results from the Mammal Survey.
No.
III. Note on Soriculus nigrescens and its subspecies. Journal
of the Bombay Natural History Society 28: 1052–1055.
inton M.A. .
b . Scientific results from the Mammal Survey.
No.
IV. The house rats of Nepal. Journal of the Bombay Natural
History Society 28: 1056–1066.
inton M.A. .
. . ry
. Report No. 37: Nepal. Bombay
Natural History Society’s Mammal Survey of India, Burma, and
Ceylon. Journal of the Bombay Natural History Society 29: 399–428.
od son . .
. On the Mammalia of Nepal. Journal of the
Asiatic Society of Bengal 1: 335–349.
od son . .
. On the Mammalia of Nepaul. Proceedings of
the Zoological Society of London 95–99.
od son . .
. Synopsis of the Vespertilionidae of Nepal.
Journal of the Asiatic Society of Bengal 4: 699–701.
od son . .
a . Synoptical description of sundry new animals,
enumerated in the Catalogue of Nip lese Mammals. Journal of the
Asiatic Society of Bengal 5: 231–238.
od son . .
b . Notice of seven species of Vespertilionidae
observed in the Central Region of Nep l. Proceedings of the
Zoological Society of London, 46pp.
Thapa
od son . .
. Classified catalogue of Nepalese Mammalia.
Annals and Magazine of Natural History 1: 152–154.
od son . .
. On the common Hare of the Gangetic Provinces,
and of the Sub-Himalaya; with a slight notice of a strictly Himalayan
species. Journal of the Asiatic Society of Bengal 9: 1183–1186.
od son . .
a . New species of Rhizomys discovered in Nepal.
Calcutta Journal of Natural History 2: 60–61.
od son . .
b . Classified catalogue of mammals of Nepal,
corrected to end of 1840, first printed in 1832. Calcutta Journal of
Natural History 2: 212–221.
od son . .
c . Notice of the Marmot of the Himalaya and of
Tibet. Journal of the Asiatic Society of Bengal 10: 777–778.
od son . .
d . Of a new species of Lagomysinhabiting Nepal,
(with Plate,) - Lagomys Nepalensis, Nob. Journal of the Asiatic
Society of Bengal 10: 854–855.
od son . .
. Classified catalogue of mammals of Nepal.
Journal of the Asiatic Society of Bengal 10: 907–916.
od son . .
.Notice of two marmots inhabiting respectively
the plains of Tibet and the Himalayan slopes near the snows, and
also of a Rhinolophus of the central region of Nepal. Journal of the
Asiatic Society of Bengal 12: 409–414.
od son . .
a . Summary description of two new species of
flying squirrel. Journal of the Asiatic Society of Bengal 13: 67–68.
od son . .
b . Classified Catalogue of Mammals of Nepal,
(corrected to the end of 1841 1843 , first printed in 1832). Calcutta
Journal of Natural History 4: 284–294.
od son . .
. On the rats, mice, and shrews of the central
region of Nepal. Annals and Magazine of Natural History 15: 266–
270.
od son . .
. On a new species of Plecotus. Journal of the
Asiatic Society of Bengal 16: 894–896.
od son . .
a . On a new Lagomys and a new Mustela
inhabiting the north region of Sikim and the proximate parts of
Tibet. Journal of the Asiatic Society of Bengal 26: 207–208.
od son . .
b . Description of a new species of Himalayan
mole Talpa macrura. Journal of the Asiatic Society of Bengal 27: 176.
o mann . .
. A review of the systematics and distribution
of Chinese red-toothed Shrews (Mammalia: Soricinae). Acta
Theriologica Sinica 7: 100–139.
ors eld .
. Brief notices of Mammalia in Nepal. Annals and
Magazine of Natural History16: 101–114.
erer .
. Order Soricomorpha. In: Wilson D.E. & D.M.
Reeder (eds.). Mammal Species of the World. A Taxonomic and
eographic eference, rd Edition. The Johns Hopkins University
Press, Baltimore, 2142pp.
n les .M. P. . e ton M. . . ands
. . . o den
.
The first record of a rare murine rodent Diomys and further records
of three shrew species from Nepal. Bulletin of the British useum
(Natural History)(Zoology) 39(3): 205–211.
. IUCN Red List of Threatened Species. Version 2013.1.
www.iucnredlist.org . Downloaded on 09 July 2013.
na ali . . . . aral . ee .P. Acharya .P.
adhyay M.
Pandey M. . hrestha . oshi . . aminchhane . ri ths
A.P. hati ada . bedi
. Amin. com ilers
. The
Status of Nepal Mammals: The National Red List Series, Department
of National Parks and Wildlife Conservation, Kathmandu, Nepal,
viii 266pp.
ohnson . . . . i ley
. hon lon ya
. Mammals from
Nepal. Journal of the Bombay Natural History Society 77: 56–63.
a amichi .
. Winter Behaviour of the Himalayan Pika,
Ochotona roylei. Journal of the Faculty of Science Hokkaido
University, Series VI, Zoology 16(4): 582–594.
a amichi .
. Daily Activities and Social Pattern of two
Himalayan Pikas, Ochotona macrotisand O. roylei, Observed at
Mt. Everest. Journal of the Faculty of Science Hokkaido University,
Series VI, Zoology 17(4): 587–609.
oc
.
. Fledermause aus Nepal (Mammalia: Chiroptera).
Senckenbergiana biology 67(1–3): 37–42.
e a l .
.A. Mcneely
. Mammals of Thailand. Association
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Checklist of Nepal mammals
Thapa
for the Conservation of Wildlife. Thailand, 758pp.
Ma
ria . .
. mar Ma
ria
. Wildlife and Protected
Areas of Nepal [Resources and Management]. S. Devi. Sharanpur,
India, 427pp.
Mallon, D.P. (2008). Pantholops hodgsonii. In: IUCN 2013. IUCN Red
List of Threatened Species. Version 2013.1. www.iucnredlist.org .
Downloaded on 09 July 2013.
Marshall . . r.
. A synopsis of Asian species of Mus (Rodentia:
Muridae). Bulletin of the American useum of Natural History 158:
173–220.
Martens .
. iethammer
. Die Waldm use (Apodemus)
Nepals. Zeitschrift f r S ugetierkunde 37(3): 144–154.
Me ada . . oyas M. arada . arita . . hrestha
da
(2001). Faunal survey of small mammals in central Nepal, with
reference to the distribution of the genus Soriculus (Insectivora,
Mammalia). Biogeography 3: 33–40.
Mitchell .M.
. A checklist of Nepalese mammals (excluding
bats). S ugetierkundliche itteilungen 23: 152–157.
Mitchell .M.
a . A checklist of Nepalese bats. S ugetierkundliche
itteilungen 26(1): 75–78.
Mitchell .M.
b . The Ochotona (Lagomorpha: Ochotonidae) of
Nepal. S ugetierkundliche itteilungen 26: 208–214.
Mitchell .M.
. The Sciurid rodents (Rodentia: Sciuridae) of
Nepal. Journal of Asian Ecology 1: 21–28.
Mitchell .M.
. New records of bats (Chiroptera) from Nepal.
Mammalia 44(3): 339–342.
Mitchell .
.P no
. New mammal records from Nepal.
Journal of the Bombay Natural History Society 73(1): 54–58.
Mitchell .
. P no
. Ectoparasites of bats from Nepal.
Journal of the Bombay Natural History Society 73(1): 84–87.
Mitchell .M.
. . er sen
. Additional new mammal
records from Nepal.Mammalia 40(1): 55–61.
Mitchell .M.
.P n o
.Ochotona lama sp. n. (Lagomorpha:
Ochotonidae): a new pika from the Tibetan highlands of Nepal.
Mammalia 39(3): 419–422.
Mol r . . rini as l
. rini as l
. al er P. . ameer
. a i mar
. Status of South Asian Non-volant Small
Mammals: Conservation Assessment and Management Plan
(C.A.M.P.) Workshop Report. Zoo Outreach Organisation / CBSGSouth Asia, Coimbatore, India.
Mol r . . Marim th
. rini as l
. Mistry A.
tson P. . .
ates . al er .P. Priya A. . . Priya
. Status of South
Asian Chiroptera: Conservation Assessment and Management
Plan (C.A.M.P.) Workshop Report. Zoo Outreach Organisation,
Conservation Breeding Specialist Group South Asia and Wildlife
Information & Liaison Development Society, Coimbatore, India.
Mol r . P. . ameer.
a . Alticola roylei. In: IUCN 2013. IUCN
Red List of Threatened Species. Version 2013.1. www.iucnredlist.
org . Downloaded on 09 July 2013.
Mol r .
P. . ameer
b . Crocidura pergrisea. In: IUCN
2013. IUCN Red List of Threatened Species. Version 2013.1. www.
iucnredlist.org . Downloaded on 09 July 2013.
Mol r . P. . ameer
c . Mus platythrix. In: IUCN 2013. IUCN
Red List of Threatened Species. Version 2013.1. www.iucnredlist.
org . Downloaded on 09 July 2013.
Myers P. . . mith . ama . ama
. . oo man
.A
recent collection of bats from Nepal, with notes on Eptesicus
dimissus. Zeitschrift fur Saugeteirkunde-International Journal of
Mammalian Biology 65(3): 149–156.
arayan . P. e a
. li er
. Porcula salvania. In: IUCN
2013. IUCN Red List of Threatened Species. Version 2013.1. www.
iucnredlist.org . Downloaded on 09 July 2013.
6070
e ton P. . M. . . ands
. . . o den
. A collection
of small mammals from eastern Nepal. Mammalia 54(2): 239–244.
iethammer .
. Martens
. Die Gattungen attusand
Maxomys in Afghanistan und Nepal. Zeitschrift f rS ugetierkunde
40: 325–355.
li er . . .
. The distribution and status of the Hispid Hare
(Caprolagus hispidus) - with some additional notes on the Pigmy
Hog (Sus salvanius). Wildlife Preservation Trust, Jersey, Channel
Islands.
Pearch, M.J. (2011). A review of the biological diversity and distribution
of small mammal taxa in the terrestrial ecoregions and protected
areas of Nepal. Zootaxa 3072: 286.
a ada . M. arada
. Cestode parasites from some Nepalese
mountain shrews. Japanese Journal of Parasitology 44(3): 196–209.
chli er . . an der traeten . Amori .
erer . ry t fe
. i it
. Mitsain
. Apodemus sylvaticus. In: IUCN 2013.
IUCN Red List of Threatened Species. Version 2013.1. www.
iucnredlist.org . Downloaded on 09 July 2013.
Scully, J. (1887). On the Chiroptera of Nepal. Journal of the Asiatic
Society of Bengal 56: 233–259.
hrestha . .
. ammals of Nepal ith eference to those
of India, Bangladesh, Bhutan and Pakistan. Mrs. Bimala Shrestha.
Kathmandu, Nepal, 371pp.
mith A. .
A. . oyer
. Ochotona thibetana. IUCN .2010.
IUCN Red List of Threatened Species. Version 2010. 4.http://www.
iucnredlist.org/(downloaded on 28 May 2010).
mith A.
. ie
. The Mammals of China. Princeton University
Press, Princeton, New Jersey, 576pp.
al .
. .M. erhe t
. Enumeration of ammals of
Nepal. Biodiversity Profiles Project Publication No. 6. Department
of National Parks and Wildlife Conservation. Ministry of Forest and
Soil Conservation. His Majesty’s Government, Kathmandu, 218pp.
ha a A. . . ahal .P. o
. ha a
. A Review on Pikas
of Nepal. Small Mammals Conservation and Research Foundation,
New Baneshwor, Kathmandu, Nepal.
ha a . P. bedi . . in h
M. . Pearch
. The first
record of Scotozous dormeri Dobson, 1875 from Nepal with new
locality records of Pipistrellus coromandra (Gray, 1838) and P.
tenuis (Temminck, 1840) (Chiroptera: Vespertilionidae). Journal
of Threatened Taxa 4(4): 2481–2489; http://dx.doi.org/10.11609/
JoTT.o2906.2481-9
homas .
. Scientific results from the Mammal Survey. No. 44.
On a new field mouse from Nepal, with a note on the classification
of the genus Apodemus. Journal of the Bombay Natural History
Society 29: 888–889.
homas . M.A. . inton
. The mammals of the 1921 Mount
Everest Expedition. Annals and Magazine of Natural History 9:
178–186.
immins . . . on
. .
c orth
. in ian
. a
(2008). Arctonyx collaris. In: IUCN 2013. IUCN Red List of Threatened
Species. Version 2013.1. www.iucnredlist.org . Downloaded on 09
July 2013.
ei el .
. Systematische Ubersicht uber die Insektenfresser
und Nager Nepals nebst Bemerkungen zur Tiergeographie.
Ergebnisse der Forschunternehmens NepalHimalaya 3: 149–196.
ilson . .
.M. eeder eds.
. Mammal Species of the
orld, rd Edition, olume . The John Hopkins University Press,
Baltimore, MD, USA, 2142pp.
orth .M.
. . hah
.Nepal Health Survey,
.
University of Hawaii Press, Honolulu, USA. 158pp.
on on P.
. Mammals of Nepal. Resources Himalaya.
http://www.resourceshimalaya.org/content files/Mammals
sAnUmAn49e70198aa10a.pdf (downloaded on 2 January 2011).
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
Thapa
Sanjan Thapa
Checklist of Nepal mammals
irrel Dremomys lokriah
Sanjan Thapa
ma e . ran e bellied imalayan
irrel Petaurista petaurista
© Bishnu Devkota
ma e . ed iant lyin
© Bishnu Timilsina
ma e . imalayan Marmot Marmota himalayana
© Arjun Thapa
© Hari Prasad Sharma
ma e . Mas ed Palm i et Paguma larvata
ma e . ed Panda Ailurus fulgens
ma e . ar e eared Pi a c o ona
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
acro s
6071
Thapa
ma e . Mo ntain
Raju Acharya
© Hem Bahadur Katuwal
Checklist of Nepal mammals
easel Mustela altaica
bellied
easel Mustela kathiah
ma e
. ishin
at Prionailurus viverrinus
ma e
. imalayan at a us
ma e
Sanjan Thapa
Sanjan Thapa
ma e . ello
© Sagar Dahal
© Hem Bahadur Katuwal
ma e . ndian are Lepus nigricollis
. east eaf nosed at Hipposideros cineraceus
c oris
Sanjan Thapa
Threatened Taxa
ma e
6072
. rab eatin Mon oose Herpestes urva
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6061–6072
o rnal of hreatened a a
eo
ee
alramliana
.threatenedta a.or
am el alron n a
ly
aln ntl an a
c/o Lee Kong Chian Natural History Museum, National University of Singapore, 6 Science Drive 2, #03-01, Singapore
117546
2
Department of Zoology, Pachhunga University College, Aizawl, Mizoram 796001, India
3,4
Department of Environmental Science, Mizoram University, Aizawl, Mizoram 796004, India
1
heokhee.ng gmail.com (corresponding author), 2 lrl zoo yahoo.co.in, 3 samuellrna@gmail.com,
4
tluanga 249 rediffmail.com
1
ISSN
Online 0974–7907
Print 0974–7893
P
A
Abstract Eutropiichthys cetosus, a new species of schilbeid ca ish is described from the Kaladan River drainage in Mizoram, northeastern
India. It can be distinguished from congeners in having a combination of the following characters: 49–52 total vertebrae, snout moderately
rounded in lateral and slightly trilobed in dorsal views, fleshy narial flap not extending medially much past medial margin of naris, mouth
rictus reaching vertical through middle of orbit, 25–35 rakers on the first gill arch, rough anterior edge of pectoral spine, 13–15 branched
pectoral-fin rays, body depth at anal-fin origin 17.5–23.5 % SL, 43–49 branched anal-fin rays, and caudal peduncle depth 7.8–8.6 % SL. A
revised key to the genus is provided.
ey ords Kaladan River drainage, Mizoram, Ostariophysi, Siluriformes.
http://dx.doi.org/10.11609/JoTT.o3883.6073-81 | oo an urn:lsid:zoobank.org:pub:DF18D869-2259-4E60-90E6-151111A908A4
ditor Neelesh Dahanukar, IISER, Pune, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3883 | Received 17 December 2013 | Final received 18 June 2014 | Finally accepted 06 July 2014
itation Ng, H.H., Lalramliana, S. Lalronunga & Lalnuntluanga (2014). Eutropiichthys cetosus, a new riverine ca ish (Teleostei: Schilbeidae) from northeastern
India. Journal of Threatened Taxa 6(8): 6073–6081; http://dx.doi.org/10.11609/JoTT.o3883.6073-81
o yri ht © Ng et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin SLRN is funded by an MZU-UGC Research Scholar’s Fellowship.
om etin nterest The authors declare no competing interests.
A thor ontrib tion LRL, SLRN and LNT collected the material, provided habitat photos and edited the manuscript. HHN gathered the data and drafted the
manuscript.
A thor etails H
H N works on the taxonomy and phylogeny of Asian ca ishes, particularly those of the superfamily Sisoroidea. L
is an Assistant Professor and his field of specialization is fish and fisheries. He is presently engaged in molecular characterization and phylogeny of freshwater fishes of
Mizoram. S
L
is a research scholar, registered for a PhD degree. He is working on diversity of fishes of Mizoram, northeastern India and their
phylogenetic analysis. L
is an Associate Professor and his field of specialization is biodiversity. He is presently engaged in taxonomy and systematics
of freshwater organisms of Mizoram.
Ac no led ements We thank the following for access to material under their care: Mark Sabaj P rez (ANSP), David Catania (CAS, CAS-SU), Karsten Hartel (MCZ),
Douglas Nelson (UMMZ), Lynne Parenti (USNM), and Kelvin Lim (ZRC). Funding for SLRN from MZU-UGC Research Scholars’ Fellowship is also acknowledged here.
Eutropiichthys cetosus - ne ri erine ca ish
et al.
The Old World ca ish family Schilbeidae is a
moderately diverse group comprising 65 species in
14 genera (Ferraris 2007; Ferraris & Vari 2007; Ng &
Vidthayanon 2011), of which approximately half (32
species) is known from Asia. The genus Eutropiichthys
Bleeker, 1862, comprises medium-sized riverine species
known from the Salween River drainage in western
Thailand, westwards to the Indus River drainage
in Pakistan. The genus has been recently revised
(Ferraris & Vari 2007) and it comprises five species: E.
britzi Ferraris & Vari, 2007 from the Irrawaddy River
drainage in Myanmar; E. burmannicus Day, 1877 from
the Irrawaddy and Sittang river drainages in Myanmar,
and the Salween River drainage in Myanmar and
western Thailand; E. murius (Hamilton, 1822) from the
Ganges-Brahmaputra River system in Bangladesh, India
and Nepal; E. salweenensis Ferraris & Vari, 2007 from
the lower Salween River drainage in western Thailand;
and E. vacha (Hamilton, 1822) from the Indus River
drainage in Pakistan, Ganges-Brahmaputra River system
in Bangladesh, Bhutan, India and Nepal, Mahanadi River
drainage in India, and Surma-Meghna River system in
Bangladesh.
During recent ichthyological surveys of the Kaladan
River drainage in Mizoram, India, the second and third
authors collected specimens of Eutropiichthys. Attempts
to identify the specimens and detailed comparison of
this material with congeners, revealed it to be a new
species, described herein as Eutropiichthys cetosus. A
revised key to the genus incorporating the results of this
study is also provided.
MA
A A
M
Measurements were made point to point with dial
calipers and data recorded to tenths of a millimeter.
Counts and measurements were made on the left side of
specimens whenever possible. Vertebrae and medianfin rays were counted from radiographs, while pairedfin rays were counted under a binocular dissecting
microscope. Subunits of the head are presented as
proportions of head length (HL). Head length and
measurements of body parts are given as proportions of
standard length (SL). Measurements and counts follow
those of Ferraris & Vari (2007) with the exception of the
gill raker counts, which are expressed as epibranchial
(upper limb) ceratobranchial (lower limb) total, and
the additions of the head depth (measured at the base
of the supraoccipital process), snout to pectoral-fin
spine base (measured from the tip of the snout to the
base of the pectoral-fin spine), dorsal-fin base length
(measured from the base of the dorsal-fin spinelet to
the base of the last dorsal-fin ray) and the body depth
at dorsal-fin origin (measured immediately anterior to
the base of the first dorsal-fin spinelet). Numbers in
parentheses following a particular meristic count are the
number of individuals with that count. Asterisks after
meristic counts indicate values for holotype. Material
examined in this study is deposited in the following
institutions: Academy of Natural Sciences of Drexel
University, Philadelphia (ANSP); California Academy
of Sciences, San Francisco (CAS, CAS-SU); Museum of
Comparative Zoology, Harvard University, Cambridge
(MCZ); Pachhunga University College Museum of Fishes,
Aizawl (PUCMF); University of Michigan Museum of
Zoology, Ann Arbor (UMMZ); National Museum of
Natural History, Smithsonian Institution, Washington DC
(USNM); and Lee Kong Chian Natural History Museum,
Singapore (ZRC).
u ro iic
s ce osus s . no .
ma es
a
urn:lsid:zoobank.org:act:35BB016E-824F-4B4E-89E4-6FF2E71A13DD
y e material
Holotype: PUCMF13024, 16.viii.2011, 121.8mm SL,
22023’1.4 N & 92057’39.3 E, vicinity of Kawlchaw Village,
Lawngtlai District, Mizoram, India, coll. S. Lalronunga.
Paratypes: PUCMF13025 (5), 91.4–127.7 mm SL,
data as for holotype.
ia nosis
Eutropiichthys cetosus can be distinguished from all
congeners, except for E. burmannicus, in having more
rakers (25–35 vs. 15–20) on the first gill arch. It differs from
E. burmannicus in the shape of the snout in both lateral
(moderately rounded in E. cetosus sp. nov. vs. distinctly
pointed in E. burmannicus; Image 2) and dorsal (slightly
trilobed in E. cetosus sp. nov. vs. acutely angular in E.
burmannicus; Images 2, 3) views, a deeper head relative
to its length (68.7–77.1% HL vs. 65.4–67.5%HL),fewer
branched pectoral-fin rays (13–15 vs. 15–17, rarely 15),
and a more slender body (depth at dorsal-fin origin
19.2–23.5% SL vs. 23.7–25.3%SL; depth at anal-fin origin
17.5–23.5% SL vs. 23.2–26.3%SL; compare Images 1
and 3). The following unique combination of characters
serves to further distinguish E. cetosus sp. nov. from
congeners: 49–52 total vertebrae, fleshy narial flap not
extending medially much past medial margin of naris,
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
et al.
© Heok Hee Ng
Eutropiichthys cetosus - ne ri erine ca ish
ma e . u ro iic
s ce osus s . no . P
M
holoty e
mouth rictus reaching vertical through middle of orbit,
rough anterior edge of pectoral spine, 43–49 branched
anal-fin rays, and caudal peduncle depth 7.8–8.6 % SL.
escri tion
General shape and appearance as in Image 1.
Biometric data in Table 1. Body elongate, compressed.
Body depth greatest at dorsal-fin origin. Dorsal profile
of body nearly straight from rear of head to dorsal-fin
origin and gently convex between posterior terminus of
dorsal-fin base and caudal-fin origin. Ventral profile of
body convex to anal-fin origin, then straight along base
of anal fin. Vent located slightly anterior of anal-fin
origin. Lateral line complete, midlateral, and extending
onto basal fleshy portion of dorsal lobe of caudal fin, with
short secondary branches extending obliquely above
and below entire length of main portion of system. Total
vertebrae 49 (1), 50 (2), 51 (2) or 52 (1).
Head compressed along entire length, subacute from
lateral view and linear from dorsal view; depth much
. mm
ndia Mi oram icinity of a lcha
illa e.
greater than width. Opercular opening broad, extending
from horizontal through anterior limit of lateral line to
vertical through middle of pupil. Opercular membranes
not connected to isthmus. Posteroventral margin
of operculum with posteriorly directed, fleshy lobe;
posterior portion of lobe rounded.
Anteriormost portion of snout subacute in lateral
view. Snout margin slightly trilobed from dorsal view
(Image 2a), but with lobes poorly defined. Anterior
naris round, anteriorly directed, and located on anterior
margin of snout. Posterior naris rounded. Posterior naris
located slightly posterodorsal and medial to anterior
naris. Width of posterior naris approximately equal
to one-half of internarial distance. Anterior margin of
naris with convex flap of skin extending medial of medial
margin of naris for distance less than transverse extent
of opening of naris.
Eye positioned laterally, visible from both dorsal and
ventral views; middle of eye positioned slightly below
horizontal through middle of vertical extent of head
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
6075
et al.
© Heok Hee Ng
Eutropiichthys cetosus - ne ri erine ca ish
a
b
. mm
© Heok Hee Ng
ma e . orsal and lateral ie s of heads of a
u ro iic
s ce osus s . no . P M
holoty e
b
ur annicus
. mm
sho in di erences in sno t sha e. ma es not to scale.
ma e . u ro iic
s ur annicus
. mm
Myanmar Mandalay.
and distinctly below horizontal through anterior naris.
Anterior and posterior portions of eye covered laterally
by connective tissue (adipose eyelid), but with ovoid,
vertically elongate opening positioned lateral to pupil.
Mouth terminal, with opening large and
posteroventrally angled. Posterior terminus of gape
6076
at vertical through middle of pupil. Lower jaw slightly
shorter than upper. Premaxillary tooth plate crescentic.
Teeth on tooth plate slender, conical, and depressible,
with approximately seven irregular rows at symphysis
that progressively reduce to three or four irregular rows
laterally. Teeth on posteromedial portion of tooth plate
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
Eutropiichthys cetosus - ne ri erine ca ish
able . iometric data for u ro iic
P
oloty e
M
et al.
s ce osus s . no . n
an e
.
Mean
Standard length (mm)
121.8
91.4–127.7
Snout to dorsal-fin
origin
35.2
32.4–35.2
33.4 1.1
Snout to anal-fin origin
47.9
45.0–48.4
46.9 1.2
Snout to pelvic-fin
insertion
36.3
36.3–38.4
37.3 0.7
Snout to pectoral-fin
spine base
22.4
20.4–22.6
21.8 0.9
Length of dorsal-fin
base
8.0
7.2–8.6
7.9 0.5
Dorsal-spine length
14.7
14.7–16.8
15.9 0.8
Length of anal-fin base
39.7
39.1–42.8
41.2 1.6
Length of first pelvicfin ray
8.0
7.3–9.1
8.3 0.8
Length of first pectoralfin ray
18.0
18.0–21.7
18.8 1.0
Length of pectoral-fin
spine
17.0
16.7–19.7
17.6 1.1
Length of dorsal
principal caudal-fin ray
25.4
21.5–27.3
24.4 2.3
Body depth at dorsalfin origin
21.3
19.2–23.5
21.5 1.7
Body depth at anal-fin
origin
20.4
17.5–23.5
21.1 2.1
Body width at pectoralfin insertion
11.3
10.5–12.6
11.5 0.7
Caudal-peduncle depth
8.1
7.8–8.6
8.3 0.3
Head length
22.6
20.4–22.6
21.3 0.7
Head depth
68.7
68.7–77.1
71.8 2.9
Interorbital distance
27.3
27.3–31.2
29.4 1.4
Individual measurements of paratypes provided in Appendix 1.
larger than remaining teeth on that plate. Outermost
teeth of upper jaw exposed laterally when mouth
closed. Accessory premaxillary tooth plate extends from
posterolateral margin of premaxillary tooth plate nearly
to rear of gape. Teeth on accessory plate arranged in
four or five irregular rows, with teeth largest medially
and progressively decreasing in size laterally. Lateral
teeth on accessory patch comparable in size to smallest
teeth on premaxilla. Palatal tooth patch in form of
parabolic arch extending posteriorly from midline to
slightly past posterior terminus of accessory tooth
patch. Anterior and lateral margins of palatal tooth
patch closely applied to, but slightly separated from,
posterior margin of premaxillary tooth patch and medial
margins of accessory tooth patch. Teeth of palatal
tooth patch slender and conical, with teeth of medial
portion of patch largest and remaining teeth becoming
progressively smaller posterolaterally. Largest teeth on
palate comparable in size to largest teeth on premaxilla.
Dentary tooth plate parabolic with slender, conical
teeth covering dorsal surface and extending onto lateral
surface of dentary. Teeth on lateral surface of dentary
visible in closed mouth. Teeth along medial portion of
anterior one-half of dentary largest with remaining
teeth becoming progressively smaller. Largest teeth on
dentary approximately equal in size to largest teeth on
premaxilla. Dentary with seven or eight irregular rows
of teeth along entire length of tooth patch. Gill rakers
on outer face of first arch 7 18 25 (1), 7 20 27 (2),
9 20 29 (1), 10 21 31 (1) or 11 24 35 (1).
Barbels in four pairs. All barbels rest in shallow
groove in skin, at least basally. Nasal barbel threadlike and extending posteriorly from lateral margin of
posterior naris to beyond vertical through posterior
limit of opercle. Maxillary barbel extends from posterior
of anterior naris to slightly past middle of pectoral fin.
Mandibular barbels in two pairs; barbel bases originate
in transverse row at level of posterior naris. Medial
and lateral mandibular barbels extend posteriorly to
transverse through pectoral-fin base.
Dorsal-fin origin located at anterior one-third of SL.
Dorsal-fin base short, about equal to length of snout.
Dorsal fin slightly smaller than pectoral fin; segmented
rays preceded by spinelet and sharply pointed, slender
spine. Spine smooth anteriorly and with 7–20 very fine
serrations along distal one-half of posterior margin, with
irregular surface but without distinct serrations along
basal portion of that margin. Fin margin straight with
rays becoming progressively shorter posteriorly; length
of last ray about one-half that of first ray. Dorsal-fin rays
II,7(6). Adipose fin small and oar-shaped, located above
posterior one-third of anal-fin base. Caudal fin deeply
forked, lobes pointed and nearly symmetrical. Outer
principal rays about three times the length of middle
rays. Principal caudal-fin rays i,7,8,i(6). Anal-fin origin
located markedly anterior to vertical through middle of
SL. Anal-fin base long. Anal-fin margin slightly concave
anteriorly, nearly straight posteriorly; posterior ray
shortest. Last fin ray without membranous connection
to caudal peduncle. Anal-fin rays v,43 (1), v,45(2), v,46
(1), v,47 (1), or v,49(1).
Pelvic fin small, its length only slightly more than
one-half that of pectoral fin. Pelvic-fin insertion located
slightly posterior of vertical through dorsal-fin origin.
Adpressed fin extending ventral of anus with tip of fin
reaching slightly past urogenital pore but falling short
of anal-fin origin. Pelvic-fin rays i,5 (6). Pectoral fin
triangular, first branched ray longest. Tip of adpressed
extends posteriorly to beyond vertical through terminus
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
6077
Eutropiichthys cetosus - ne ri erine ca ish
et al.
30
24
23
20
●
22
10
92
70
80
0
i
90
400
re . Ma sho in ty e locality of u ro iic
93
94
100
kilometers
s ce osus s . no .
of dorsal-fin base. Pectoral-fin spine slender, but more
robust than that of dorsal fin; with fine roughened ridge
anteriorly and 12–23 retrorse serrations on distal twothirds of posterior margin. Pectoral-fin rays I,13,i (3),
I,14,i (1) or I,15 (2).
highly likely to occur in parts of the river drainage that
flow through Myanmar as well. The Kaladan River (also
known as Chhimtuipui in Mizoram) originates from the
Chin Hills in Myanmar and debouches into the Bay of
Bengal near Sittwe in Myanmar. The specimens were
abitat and istrib tion
This species is currently known only from the Kaladan
River drainage in southern Mizoram (Fig. 1), although it
© Lalramliana
oloration
In 70% ethanol: Body variably brown dorsally, pale
gray on lateral and ventral surfaces, with melanophores
decreasing in density ventrally. Dorsal half of head
brown, ventral half pale gray. Dorsal and caudal fins
unpigmented other than for diffuse, marginal, dark
band. Adipose, anal and pelvic fins unpigmented.
Pectoral fin with scattered melanophores on interradial
membranes, particularly on dorsal half of fin; remainder
of fin unpigmented. Maxillary and lateral mandibular
barbels dusky on dorsal surfaces. Nasal barbel and
medial mandibular barbel unpigmented.
ma e . y e locality of u ro iic
s ce osus s . no .
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
Eutropiichthys cetosus - ne ri erine ca ish
collected from a clear, slow and moderate flowing river
with a depth of 1–4 m (Image 4).
tymolo y
The specific epithet comes from the adjectival
form of the Latin cetus’, meaning a large sea animal
(commonly referred to a whale). This name is used in
allusion to the numerous gill rakers of this species, which
are reminiscent of baleen in baleen whales.
Eutropiichthys cetosus sp. nov. is morphologically
similar to E. burmannicus, but can be distinguished from
it by the characters as outlined in the diagnosis.
Besides the higher number of gill rakers in the first
gill arch (as outlined in the diagnosis), E. cetosus sp.
nov. is further distinguished from E. britzi in having a
lesser number of vertebrae (49–52 vs. 52–54), a more
slender body (depth at anal-fin origin 17.5–23.5 % SL
vs. 23.3–27.1 % SL) and caudal peduncle (depth 7.8–
8.6 % SL vs. 9.2–11.0 % SL), and from E. murius (we
follow Ferraris & Vari 2007 in considering Pachypterus
melanurus Swainson, 1839 a junior synonym) in having
a higher number of vertebrae (49–52 vs. 43–45), a
more posteriorly-extended gape (mouth rictus reaching
vertical through middle of orbit vs. anterior orbital
margin), a rough (vs. smooth) anterior edge of the
pectoral spine, more branched pectoral- (13–15 vs. 11
or 12) and anal-fin rays (43–49 vs. 32–37). In both E.
britzi and E. murius, the fleshy flap along the anterior
margin of the posterior naris proximally reaches beyond
the medial margin of the naris by a distance equal to, or
greater than, the transverse dimension of the posterior
naris (vs. proximally reaching beyond the medial margin
of the naris for a distance distinctly less than the
transverse length of the posterior naris in E. cetosus sp.
nov.). We follow Ferraris & Vari (2007) in considering
Pseudeutropius murius batarensis a junior synonym of
E. murius, although they raised the possibility that the
two specimens on which Shrestha’s (1981) description
of P. murius batarensis is based might instead be a
species of Clupisoma. Without directly examining the
type specimens of P. murius batarensis, we are unable
to verify Ferraris & Vari’s hypothesis.
Eutropiichthys cetosus sp. nov. further differs from
E. salweenensis in having a lesser number of vertebrae
(49–52 vs. 52–54), a rough (vs. smooth) anterior edge
of the pectoral spine, a more slender caudal peduncle
(depth 7.8–8.6% SL vs. 9.2–10.2) and from E. vacha (we
et al.
follow Ferraris & Vari 2007 in considering Pachypterus
punctatus Swainson, 1839 a junior synonym) in the
shape of the snout in both lateral (moderately rounded
in E. cetosus vs. distinctly pointed in E. vacha) and dorsal
(slightly trilobed in E. cetosus sp. nov. vs. acutely angular
in E. vacha) views, and a more slender caudal peduncle
(depth 7.8–8.6% SL vs. 8.8–10.4% SL).
The Kaladan River, lying between the Surma-Meghna
River system in the north and the Chindwin-Irrawaddy
River drainage in the east, harbours high endemism of
hillstream fish fauna (Anganthoibi & Vishwanath 2010;
Dishma & Vishwanath 2012; Lokeshwor & Vishwanath
2012; Ng et al. 2013). Our discovery of a new fish species
typically found in the main channels of larger rivers in
this drainage suggests that a similar level of endemism
may also be found in the non-hillstream freshwater
ichthyofauna of the Kaladan River drainage.
om arati e material
Eutropiichthys burmannicus: CAS 88816 (1), 113.7
mm SL, Bago Region, Sittaung River at Taungoo,
18055’N & 96025’E. ZRC 43514 (5), 108.7–193.3 mm
SL, Myanmar: Mandalay Region, market in Mandalay
(Image 3). Additional data from Ferraris & Vari (2007).
E. britzi: CAS-SU 39868 (7), 186.0–229.0 mm SL,
Myanmar: Sagaing Region, market at Monywa, on
Chindwin River. Additional data from Ferraris & Vari
(2007).
E. murius: UMMZ 208724 (3), 93.3–108.1 mm SL;
Bangladesh: Sylhet District, Sharigat bazaar, 35 km NE
of Sylhet on Sylhet–Shillong highway, 2504’N & 9207’E.
UMMZ 244742 (1), 86.0mm SL, India: West Bengal
State, Mansai River, 1km after Amtala on JalpaiguriCoochbehar Road, 26019’50 N & 89014’4 E. USNM
316716 (2), 101.0–102.89 mm SL, India: Uttar Pradesh
State, Kanpur, 26028’N & 88030’E. Additional data from
Ferraris & Vari (2007).
E. salweenensis: CAS 76261 (holotype), 124.0mm
SL; Thailand: Mae Hong Son Province, Salween River
20km upriver from Mae Sam Laep. Additional data from
Ferraris & Vari (2007).
E. vacha: ANSP 85763 (1), 132.7mm SL, India: Mumbai.
CAS 61841 (2), 88.8–135.9 mm SL, India: Odisha State,
market at Sonepur, 20050’N & 83059’E. CAS 94224 (1),
212.5mm SL, India: Odisha State, Hirakud Reservoir or
Sambalpur market. MCZ 4257 (1), 113.7mm SL, UMMZ
238802 (1), 144.0mm SL; India: West Bengal State,
Kolkata. UMMZ 208293 (1), 178.8mm SL, Bangladesh:
Comilla District, Meghna River, downstream from Gumti
River mouth, 23019’N & 90038’E. UMMZ 208313 (1),
141.7mm SL, Bangladesh: Comilla District, Meghna River,
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
6079
Eutropiichthys cetosus - ne ri erine ca ish
et al.
ey to the s ecies of Eutropiichthys modi ed from erraris
ari
1.
Anal fin with 32–37 branched rays; pectoral fin with 11 or 12 branched rays (Ganges-Brahmaputra river system in Bangladesh,
India and Nepal) .............................................................................................................................................................. E. murius
Anal fin with 43–55 branched rays; pectoral fin with 13–17 branched rays ................................................................................. 2
2.
Gill rakers on first arch 22–35 ........................................................................................................................................................ 3
Gill rakers on first arch 15–20 .........................................................................................................................................................4
3.
Snout distinctly pointed and acutely angular when viewed laterally and dorsally respectively; head depth 65.4–67.5% HL;
body depth at dorsal-fin origin 23.6–25.3% SL (Irrawaddy and Sittang river drainages in Myanmar; Salween River drainage in
Myanmar and western Thailand) ............................................................................................................................ E. burmannicus
Snout moderately rounded and slightly trilobed when viewed laterally and dorsally respectively; head depth 68.7–77.5% HL;
body depth at dorsal-fin origin 19.2–23.5% SL (Kaladan River drainage in India and possibly Myanmar) ......... ce osus s . no .
4.
Fleshy narial flap extending medially past medial margin of naris, nearly reaching midline of head (Irrawaddy River drainage in
Myanmar) ........................................................................................................................................................................... E. britzi
Fleshy narial flap not extending medially much past medial margin of naris ................................................................................ 5
5.
Pectoral spine with smooth anterior margin; snout bluntly rounded in lateral view; total vertebrae 52–54 (lower Salween River
basin, in western Thailand) ..................................................................................................................................... E. salweenensis
Pectoral spine with rough anterior margin; snout distinctly pointed in lateral view; total vertebrae 49–51(Indus River drainage
in Pakistan; Ganges-Brahmaputra river system in Bangladesh, Bhutan, India and Nepal; Mahanadi River drainage in India;
Surma-Meghna river system in Bangladesh) ..................................................................................................................... E. vacha
just upstream of Chandpur at Jahasmarachar, 23015’N &
90039’E. UMMZ 208444 (1), 117.5mm SL, Bangladesh:
Barisal District, Meghna River at Gazipur Char, 22047’N
& 90043’E. UMMZ 237501 (1), 294.6mm SL; Pakistan:
Punjab Province, Jhelum River at Jhelum. USNM 165030
(1), 178.2mm SL, Pakistan: Punjab Province, Ravi River at
Lahore. Additional data from Ferraris & Vari (2007).
An anthoibi .
. ish anath
. Pseudecheneis koladynae,
a new sisorid ca ish from Mizoram, India (Teleostei: Siluriformes).
Ichthyological Exploration of Fresh aters 21(3): 199–204.
ishma M.
. ish anath
. Barilius profundus, a new
cyprinid fish (Teleostei: Cyprinidae) from Koladyne basin, India.
Journal of Threatened Taxa 4(2): 2363–2369; http://dx.doi.
org/10.11609/JoTT.o2838.2363-9
erraris . . r.
. Checklist of ca ishes, recent and fossil
(Osteichthyes: Siluriformes), and catalogue of siluriform primary
types. Zootaxa 1418: 1–628.
erraris . . r.
.P. ari
. Revision of ca ishes of the
genus Eutropiichthys, with the description of two new species
(Siluriformes: Schilbidae). Copeia 2007(4): 866–885; http://dx.doi.
org/10.1643/0045-8511(2007)7 866:ROCOTG 2.0.CO;2
o esh or .
. ish anath
.Schistura koladynensis, a new
species of loach from the Koladyne basin, Mizoram, India (Teleostei:
Nemacheilidae). Ichthyological Exploration of Fresh aters 23(2):
139–145.
. . alramliana . alron n a
aln ntl an a
.
Pseudolaguvia nubila, a new sisorid ca ish (Teleostei: Sisoridae)
from northeastern India. Zootaxa 3647(4): 518–526; http://dx.doi.
org/10.11646/zootaxa.3647.4.2
. .
. idthayanon
. Pseudeutropius indigens, a new
species of schilbeid ca ish (Teleostei: Siluriformes) from peninsular
Thailand. Zootaxa 3037: 45–50.
hrestha
.The Fishes of Nepal. Curriculum Development
Centre, Tribhuvan University, Kathmandu. xviii 318 pp.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
Eutropiichthys cetosus - ne ri erine ca ish
A
endi
et al.
. iometric data for u ro iic
P
Standard length (mm)
s ce osus s . no .
Paraty e
M
P
Paraty e
M
araty es .
P
Paraty e
M
P
Paraty e
M
P
Paraty e
M
127.7
113.0
104.7
102.4
91.4
Snout to dorsal-fin origin
33.8
32.4
33.9
32.4
32.9
Snout to anal-fin origin
46.4
47.3
46.5
45.0
48.4
%SL
Snout to pelvic-fin insertion
38.4
37.5
37.7
37
37.1
Snout to pectoral-fin spine base
21.9
20.4
21.2
22.4
22.6
Length of dorsal-fin base
7.4
8.1
7.2
8.0
8.6
Dorsal-spine length
16.8
15.5
16
16.5
16.1
Length of anal-fin base
40.7
42.5
39.1
42.4
42.8
Length of first pelvic-fin ray
7.3
7.7
9.1
9.0
8.9
Length of first pectoral-fin ray
18.3
18.2
18.9
20.7
18.4
Length of pectoral-fin spine
17.6
17.1
17.2
19.7
16.7
Length of dorsal principal caudalfin ray
21.5
27.3
24.5
26.0
21.8
Body depth at dorsal-fin origin
19.2
22.5
22.7
19.7
23.5
Body depth at anal-fin origin
20.7
22.2
22.2
17.5
23.5
Body width at pectoral-fin
insertion
11.4
12.1
10.5
11.3
12.6
Caudal-peduncle depth
7.8
8.4
8.4
8.6
8.5
Head length
21.5
20.4
21.1
21.0
21.4
Head depth
71.2
77.1
72.3
71.6
69.9
Interorbital distance
28.8
29
31.2
29.3
30.6
%HL
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6073–6081
o rnal of hreatened a a
.threatenedta a.or
ly
ISSN
Online 0974–7907
Print 0974–7893
P
o mya it ho dh ry
A
2
1
Centre for Biodiversity and Ecological Studies, P1/1B, Garia Park, Kolkata, West Bengal 700084, India;
Department of Zoology, V.J.R. College (a liated to the University of Calcutta), 8/2, Bejoygarh, Jadavpur, Kolkata,
West Bengal 700032, India
wildlifesc@gmail.com
Abstract The Indian Sundarbans, part of the globally famous deltaic eco-region, is little-studied for butterfly diversity and ecology. The
present study reports 76 butterfly species belonging to five families, which is a culmination of 73 species obtained from surveys conducted
over a period of three years (2009–2011) in reclaimed and mangrove forested areas and three species obtained from an earlier report.
Six of these species are legally protected under the Indian Wildlife (Protection) Act, 1972. Random surveys were employed for both the
study areas, supplemented by systematic sampling in reclaimed areas. The reclaimed and forested areas differed largely in butterfly
richness (Whittaker’s measure of diversity 0.55). For sample-based rarefaction curves, butterfly genera showed a tendency to reach an
asymptote sooner than the species. Numerous monospecific genera (77.19% of the taxa) resulted in a very gentle but non-linear positive
slope for the species-genus ratio curve. A species-genus ratio of 1.33 indicated strong intra-generic competition for the butterflies of the
Indian Sundarbans. Mangrove areas were species poor, with rare species like Euploea crameri, Colotis amata and Idea agamarshchana
being recorded in the mangrove area; while Danaus genutia was found to be the most frequent butterfly. Butterfly abundance was very
poor, with no endemic species and the majority (53.9% of the taxa; n 41) were found locally rare. The changing composition of butterflies
in the once species-poor mangrove zone of the fragile Sundarbans may interfere with their normal ecosystem functioning.
ey ords Asclepiadaceae, butterfly, Danaus genutia, India, Mangrove, reclaimed area, Sundarbans.
http://dx.doi.org/10.11609/JoTT.o3787.6082-92 | oo an urn:lsid:zoobank.org:pub:4F1E7FBB-0BEA-4A43-AED1-2372A5AF57D8
ditor B.A. Daniel, Zoo Outreach Organisation, Coimbatore, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3787 | Received 28 September 2013 | Final received 05 July 2014 | Finally accepted 08 July 2014
itation Chowdhury, S. (2014). Butterflies of Sundarban Biosphere Reserve, West Bengal, eastern India: a preliminary survey of their taxonomic diversity, ecology
and their conservation. Journal of Threatened Taxa 6(8): 6082–6092; http://dx.doi.org/10.11609/JoTT.o3787.6082-92
o yri ht © Chowdhury 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin Centre for Biodiversity and Ecological Studies Ref No: CBES/2010/S-PI)
om etin nterest The author declares to have financial support from the Centre for Biodiversity and Ecological Studies (CBES) for the submitted work, and no
other relationships or activities that have inspired the same.
A thor etails D . S
C
is a faculty member of the Department of Zoology, V.J.R. College, Kolkata and founder member of Centre for Biodiversity and Ecological Studies (CBES). He has been working on the biodiversity and ecology of Indian insects and authored several research articles. He also initiated
BIOME india’, an online resource on the ecosystem-based conservation programmes of India.
Ac no led ement The author is indebted to the Centre for Biodiversity and Ecological Studies for supporting, in part, the survey work on lepidopteran fauna
and different mangrove and mangrove-associated floral components in Indian Sundarbans. Nihar Mondal, Tanmoy Mondal and Amit Das among several others
deserve special thanks for logistic support during field trips in different parts of central, western and eastern Sundarbans.
er ies of
ndarban ios here eser e
Sundarbans forms a unique eco-region in the vast
deltaic region on the Bay of Bengal, hosting one of
the most extensive mangrove forests of the world,
spreading over India and Bangladesh. This region
is also the only mangrove tiger land in the world,
securing the endangered Royal Bengal Tiger Panthera
tigris tigris populations with inimitable cases of tigerhuman conflict. The human populations in this fragile
eco-region have sustained themselves by harvesting
forest products and by reclaiming the forest land for
agricultural purposes, over the last two centuries. The
usual flushing of tidal water and natural inundation has
been greatly affected by embankment constructions in
the Sundarbans, resulting in quick succession or shifting
in plant communities (Sanyal et al. 1984).
Butterflies are recognized as focal species of
conservation in several areas of the world (New 2011).
These widely studied insects show significant ecological
contributions in different ecosystems through herbivory
and pollination services. The Indian Sundarbans, being a
unique deltaic region with a rich biorepository, suffered
from inadvertent reports on butterflies, accordingly
lacking a detailed survey for the same. Literature
reviews indicated a meagre eight species (Mandal &
Nandi 1989; Mandal & Maulik 1997; Kehimkar 2008)
to be precisely reported from this region. Hence, an
inventory on the butterfly fauna in Indian Sundarbans
and their mode of habitat association was required for
this globally important eco-region.
MA
A
A
M
t dy ite
The Indian Sundarbans, comprising an area of
9,630km2 is part of the delta of the Ganga-BrahmaputraMeghna basin in Asia; and along with the Bangladesh
Sundarbans hosts one of the largest continuous
mangrove forests in the world. The landscape is
characterized by a web of tidal water systems. Only the
Hugli and Ichamati-Raimangal rivers carry freshwater
flow of some significance (Danda et al. 2011). The Indian
Sundarbans are located between 21031’–22030’N and
88010’–89051’E, spreading over the southern parts in the
districts of North and South 24-Parganas of the state of
West Bengal (Naskar & Mandal 1999) and demarcated
by the Dampier and Hodges Line’ in the north. This area
has received the status of Sundarbans Biosphere Reserve
(SBR) in 1989, by being the world’s first mangrove forest
ho dh ry
to be brought under scientific management. Mangrove
forests cover 4,264km2 and include a tiger reserve
covering 2,585km2, a national park covering 1,330km2
and three wildlife sanctuaries, namely Sajnekhali WS
(362km2), Lothian Island WS (39km2) and Halliday Island
WLS (5.8km2). The remaining area, covering 5,366km2,
comprises reclaimed land (Chaudhuri & Choudhury
1994; Zockler et al. 2005).
The region experiences a humid, tropical, maritime
climate, with an average annual rainfall of 142.5mm.
Mean annual maximum and minimum temperatures
are about 300C and 230C respectively. Humidity is high,
80% on average due to proximity to the Bay of Bengal
(Chowdhury 2011). Recent reports suggest that air
temperature over the Sundarbans and adjacent parts of
Bay of Bengal are gradually increasing (Huq et al. 1999;
Agrawala et al. 2003).
Based on the vegetation pattern and extent of
exposure to saline water regimes, two broad categories
of eco-zones were recognized among the 18 sites that
were sampled in the eastern, central and western
Sundarbans (Fig. 1): 1 - Mangrove area and 2 - Reclaimed
area. The former is exposed to saline tidal submergence
with luxuriant mangrove vegetation (Image 1), while the
latter is retained for human settlements and agricultural
practices with low saline influence and non-mangrove
vegetation (Image 2).
er y sam lin and data analyses
Butterflies were sampled by visual estimation surveys
for three years: 2009–2011; with samplings repeated
for pre-monsoon, monsoon and post-monsoon months
of the study period. Sampling of butterflies varied
according to habitat patterns over the deltaic region. In
the mangrove forested regions, butterflies were broadly
sampled in a random manner along the forest edges
and trails available. Whereas in the reclaimed areas,
surveys were carried out especially in the greener areas
(viz., gardens, agricultural fields, fragmented wooded
areas) so as to encounter butterflies more frequently
as compared to the highly degraded and modified
locales. Both random searches and systematic sampling
(along definite trails) were carried out in the latter.
Butterflies were recorded from 0800–1700 hr each day.
Species were observed while perched on surrounding
vegetation, in flight and puddling as well as nectar
feeding were recorded and photographed in situ for
reference. Species identifications were done following
available literature (Evans 1932; Wynter-Blyth 1957;
Kehimkar 2008). Taxonomic classification of butterfly
families was followed after van Nieukerken et al. (2011).
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
er ies of
ndarban ios here eser e
ho dh ry
N
A
A
ol ata
1 - Hasnabad (N 2 Pgs)
2 - Baharu (S 24 Pgs)
3 - Bakkhali I (S 24 Pgs)
4 - Bali (S 24 Pgs)
8 - Pakhirala (S 24 Pgs)
ly R
ogh
7 - Koikhali I (S 24 Pgs)
Ho
6 - Canning (S 24 Pgs)
.
5 - Basanti (S 24 Pgs)
9 - Sagar Is (S 24 Pgs)
10 - Jhingakhali (N 2 Pgs)
11 - Bakkhali II (S 24 Pgs)
12 - Burirdabri (S 24 Pgs)
13 - Jharkhali (S 24 Pgs)
14 - Koikhali II (S 24 Pgs)
15 - Lothian Is (S 24 Pgs)
16 - Lothian Is (S 24 Pgs)
la R
Mat
Thak
uran
18 - Sudhanyakhali (S 24 Pgs)
.
R.
17 - Sajnekhali (S 24 Pgs)
A
Mangrove Forest
A
Recliamed Area
Dampier-Hodges Line
0
10
20 km
International Boundary
i re . A ma of the ndian
state of est en al ndia.
ndarbans delta sho in the sam lin sites encom assin the orth and o th
The presence-absence data for butterflies in the
sampling sites in the western, central, and eastern
Sundarbans were pooled to form a species sample
incidence matrix. Taxon sampling curves (samplebased rarefaction curves) for butterfly species and for
the genera to which they belong were generated using
Par anas districts of the
Estimate S version 9.1.0 (Colwell 2013); their speciesto-genus ratios were plotted along with so as to deduce
levels of competitive interactions among species within
genera (Simberloff 1978; J rvinen 1982).
Estimation of
-diversity (diversity between
habitats) between the mangrove forests and reclaimed
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
ndarban ios here eser e
ho dh ry
ma e . Man ro e forest at
dhanya hali in
ndarbans ndia.
areas of the Sundarban deltaic region were done using
Whittaker’s measure W (Whittaker 1960) for presence/
absence data (as in the present case):
S/
.
.. (1)
W
where S the total number of species recorded in
both sites, and
the average sample richness. The
obtained result was calculated on the 0 (minimum
diversity) to 1 (maximum diversity) scale by subtracting
1 from the obtained answer (Magurran 2004).
Frequency of occurrence (FO) of the butterfly species
was calculated by considering the number of sampled
sites in which the species occurred in relation to the total
number of samples (or sites) surveyed (Severiano et al.
2012). Such an analysis was viewed into the following
categories when FO 70% very frequent (VF) ; 70%
– 45% frequent (F); 45% – 15% Infrequent (IF) ;
15% Rare .
A total of 76 species of butterflies have been
reported hitherto from Indian Sundarbans (Table 1).
Of these, 73 species were recorded following the three
years (2009–2011) field survey. The remaining three
species, viz., raphium nomius, Eurema brigitta and
Idea agamarschana, were recorded by Mandal & Nandi
(1989) in Sundarbans but not recorded in the present
survey. All the following analyses have been done with
respect to the 76 species.
Among the five recorded butterfly families in
Sundarbans, Nymphalidae appears to be most species
rich (n 24, 31.6%), followed by Lycaenidae (n 19, 25%).
The family with lowest species richness is Papilionidae
(n 9, 11.8%).
Pieridae and Hesperiidae were
intermediate with 17.1% (n 13) and 14.5% (n 11) of the
Soumyajit Chowdhury
Soumyajit Chowdhury
er ies of
ma e . eclaimed area at asanti in
ndarbans ndia.
total species. Regarding genus richness, the butterfly
families followed nearly the same trend as their species;
nymphalids (n 16, 28%) outnumber the others while
papilionids ranked last (n 3, 5.2%) in the list (Table 2).
The distribution of butterfly species across genera
was found to be highly skewed. A large proportion of
genera (44 out of 57) recorded in the present study were
represented by single species, whereas the remaining
eight genera by two species, four genera by three
species and one genus by four species (Fig. 2). Figure 3
illustrates the most prevalent genera, that is, those with
three and four species.
Figure 4 depicts sample-based species and genus
rarefaction curves for the butterfly dataset. Both the
curves do not reach a plateau with increasing sample
numbers. However, the number of genera shows
a tendency to reach an asymptote sooner than the
number of species. Moreover, a very low speciesgenus ratio (S/G 1.33) exists for the butterflies of Indian
Sundarbans delta.
A fairly high beta diversity for the butterfly fauna
was indicated by the obtained value ( W 0.55) of the
Whittaker’s measure between the mangrove forests
and reclaimed areas of Indian Sundarbans. All the
butterfly families show a higher species richness in the
reclaimed areas than in the mangrove forest zones (Fig.
5). Whereas Nymphalidae, Lycaenidae and Hesperiidae
fall away by about 50% in mangroves as compared
to reclaimed areas, a marked drop can be seen in
Lycaenidae and Hesperiidae (by 20%) in mangrove
forests as against reclaimed areas.
Of the 76 species of butterflies, most are common’
and generalist’ as none of the species are threatened
globally as per the IUCN Red List (2011). However,
regarding their frequency of occurrence in the
Sundarbans, the majority of butterflies were classified as
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
6085
er ies of
ndarban ios here eser e
ho dh ry
able . A s ecies list of the b er y fa na in ndian ndarbans. he referential occ rrences of the s ecies are indicated by s eci c eco
ones in hich they ere recorded bein abbre iated as M man ro e forest and A reclaimed non forest area
a a
cc rrence
a a
FO
cc rrence
FO
Superfamily Papilionoidea
Subfamily Pierinae
Family Papilionidae
26
Common Albatross Appias albina
(Boisduval, 1836)
RA
R
27
Striped Albatross Appias libythea
(Fabricius, 1775)
RA, MF
IF
28
Small Salmon Arab Colotis amata
(Fabricius, 1775)
RA
R
29
Common Gull Cepora nerissa (Fabricius,
1775)
RA, MF
R
30
Common Jezebel Delias eucharis (Drury,
1773)
RA, MF
IF
31
Yellow Orange Tip Ixias pyrene (Linnaeus,
1764)
RA
R
32
Psyche Leptosia nina (Fabricius, 1793)
RA
IF
33
Common Wanderer Pareronia valeria
(Cramer, 1776)
RA
R
RA
R
Subfamily Papilioninae
1
Common Jay raphium doson (C & R
Felder, 1864)
RA
IF
2
Tailed Jay Graphium agamemnon
(Linnaeus, 1758)
RA
IF
3
Lime Papilio demoleus (Linnaeus, 1758)
RA, MF
IF
4
Common Mime Papilio clytia (Linnaeus,
1758)
RA, MF
R
5
Blue Mormon Papilio polymnestor (Cramer,
1775)
RA
R
6
Common Mormon Papilio polytes
(Linnaeus, 1758)
RA, MF
F
7
Common Rose Pachliopta aristolochiae
(Fabricius, 1775)
RA, MF
IF
8
Crimson Rose Pachliopta hector (Linnaeus,
1758) +
RA, MF
IF
Subfamily Curetinae
9
Spot Swordtail raphium nomius (Esper,
1798)
MF
R
34
Family Lycaenidae
Indian Sunbeam Curetis thetis (Drury,
1773)
Family Hesperiidae
Subfamily Polyommatinae
Subfamily Coeliadinae
35
Gram Blue Euchrysops cne us (Fabricius,
1798)
RA
IF
36
Lime Blue Chilades laius (Stoll, 1780)
RA
R
37
Pea Blue Lampides boeticus (Linnaeus,
1767)
RA
IF
38
Common Cerulean Jamides celeno
(Cramer, 1775)
RA
R
RA
R
10
Brown Awl Badamia exclamationis
(Fabricius, 1775)
RA
R
Subfamily: Hesperiinae
11
Chestnut Bob Iambrix salsala (Moore,
1866)
12
Indian Bob Palm Suastus gremius
(Fabricius, 1798)
RA
R
39
13
Dark Palm Dart Telicota ancilla (HerrichSch ffer, 1869)
Forget-me-not Catochrysops strabo
(Fabricius, 1793)
RA
R
40
RA
IF
14
Common Dartlet Oriens gola (Moore,
1877)
Dark Grass Blue Zi eeria karsandra
(Moore, 1865)
RA
R
41
RA
R
15
Grass Demon daspes folus (Cramer, 1775)
Lesser Grass Blue Zi ena otis (Fabricius,
1787)
RA
R
42
IF
RA
R
Tiny Grass Blue Zizula hylax (Fabricius,
1775)
RA
16
Common Red Eye Matapa aria (Moore,
1866)
Common Straight Swift Parnara guttata
(Bremer and Grey, 1852)
43
R
RA, MF
IF
Common Lineblue Prosotas nora (C & R
Felder, 1860)
MF
17
18
Rice Swift Borbo cinnara (Wallace, 1866)
RA
IF
44
Common Pierrot Castalius rosimon
(Fabricius, 1775)
RA
R
19
Small Branded Swift Pelopidas mathias
(Fabricius, 1798)
RA, MF
IF
45
Rounded Pierrot Tarucus nara (Kollar,
1848)
RA
R
46
Quaker Neopithecops almora (Butler,
1870)
RA
IF
RA
R
Subfamily Pyrginae
20
Common Snow Flat Tagiades apetus (Stoll,
1781)
RA
R
Family Pieridae
Subfamily Coliadinae
21
Common Emigrant Catopsilia pomona
(Fabricius, 1775) +
RA
R
22
Mottled Emigrant Catopsilia pyranthe
(Linnaeus, 1758)
RA, MF
F
23
Common Grass Yellow Eurema hecabe
(Linnaeus, 1758)
RA, MF
F
24
Small Grass Yellow Eurema brigitta
(Cramer, 1780)
MF
R
25
Three-spot Grass Yellow Eurema blanda
(Boisduval, 1836)
RA
R
6086
Subfamily Theclinae
47
Common Red Flash apala iarbus
(Fabricius, 1787)
RA
R
48
Slate Flash Rapala manea (Hewitson,
1863)
RA
R
49
Monkey Puzzle Rathinda amor (Fabricius,
1775)
RA
R
50
Falcate Oak Blue Mahathala ameria
(Hewitson, 1832)
RA
R
51
Common Silverline Spindasis vulcanus
(Fabricius, 1775)
RA
R
52
Yamfly Loxura atymnus (Stoll, 1780)
RA
R
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
ndarban ios here eser e
a a
ho dh ry
cc rrence
50
FO
Family Nymphalidae
Angled Castor Ariadne ariadne (Linnaeus,
1758)
RA
R
54
Common Castor Ariadne merione (Cramer,
1779)
RA
IF
Common Indian Crow Euploea core
(Cramer, 1780)
RA, MF
F
56
Spotted Black Crow Euploea crameri
(Lucas, 1853) +
RA, MF
R
57
Blue Tiger Tirumala limniace (Cramer,
1775)
RA, MF
F
58
Plain Tiger Danaus chrysippus (Linnaeus,
1758)
RA, MF
F
59
Striped Tiger Danaus genutia (Cramer,
1779) +
RA, MF
F
60
White Tiger Danaus melanippus (Cramer,
1777) +
RA, MF
IF
61
Tree Nymph Idea agamarshchana (C and R
Felder, 1865) +
MF
R
re
53
ency
Subfamily Biblidinae
Subfamily Danainae
RA
R
1
0.5
Subfamily Nymphalinae
RA, MF
IF
68
Grey Pansy Junonia atlites (Linnaeus, 1763)
RA, MF
IF
69
Lemon Pansy Junonia lemonias (Linnaeus,
1758)
RA
R
70
Peacock Pansy Junonia almana (Linnaeus,
1758)
RA
IF
RA, MF
IF
RA
R
RA
IF
RA, MF
F
Subfamily Satyrinae
ycalesis perseus
71
Common Bushbrown
(Fabricius, 1775)
72
Dark-Brand Bushbrown
(Linnaeus, 1758)
ycalesis mineus
73
Common Evening Brown
(Linnaeus, 1758)
elanitis leda
74
Common Palmfly Elymnias hypermenstra
(Linnaeus, 1758)
75
Common Five Ring pthima baldus
(Fabricius, 1775)
RA
R
76
Common Four Ring Ypthima huebneri
(Kirby, 1871)
RA
R
0
Graphium
Great Eggfly Hypolimnas bolina (Linnaeus,
1758)
enera
i re . Most s ecies rich enera of b er ies of ndarbans
com risin of t o or more s ecies hitherto recorded from this
re ion.
Species/Genus (x100)
140
120
100
a a
67
1.5
Danaus
IF
Ypthima
RA
2
Junonia
IF
Euploea
RA
2.5
Ariadne
Subfamily Limenitinae
3
Mycalesis
IF
Rapala
RA
3.5
Eurema
Common Leopard Phalanta phalantha
(Drury, 1773)
Common Sailer Neptis hylas (Moore, 1758)
4
4.5
Catopsilia
63
66
2
3
mber of s ecies er en s
Appias
IF
odu a procris (Cramer,
1
i re . re ency of the n mber of b er y s ecies recorded for
each en s re orted from ndian ndarbans. he total n mber of
obser ation in each cate ory is sho n on to of the bar. Most of the
enera ere re resented by sin le s ecies.
mber of s ecies er en s
RA
Commander
1777)
1
4
4
Tawny Coster Acraea violae (Fabricius,
1775)
65
8
0
62
Common Baron Euthalea aconthea
(Cramer, 1777)
20
10
Subamily Heliconiinae
64
30
Prachliopta
55
44
40
Papilio
er ies of
80
Species
60
Genera
40
FO Frequency of Occurrence, VF Very Frequent, F Frequent, I Infrequent,
R Rare
Species reported in Mandal and Nandi (1989)
+
Species reported from both literature reviews and field surveys
20
0
i
b
0
6
am les
12
18
re . a on sam lin c r es for s ecies and for the enera of
er y fa na of ndian ndarbans delta to hich they belon
ith the s ecies en s ratio.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
6087
er ies of
ndarban ios here eser e
ho dh ry
able . er ie of the ta onomic di ersity of b
ndarbans.
amily
bfamily
enera
er ies of
ecies
Papilionidae
1 (6.7%)
3 (5.2%)
9 (11.8%)
Hesperiidae
3 (20%)
11 (19.3%)
11 (14.5%)
Pieridae
2 (13.3%)
9 (15.7%)
13 (17.1%)
Lycaenidae
3 (20%)
18 (31.7%)
19 (25.0%)
Nymphalidae
6 (40%)
16 (28.1%)
24 (31.6%)
15 (100%)
57 (100%)
76 (100%)
otal 5
able . e ally rotected b er ies of ndarbans nder the
ndian ildlife Protection Act
PA .
PA ched le
Schedule I
er y family
ecies
Papilionidae
Pachliopta hector (Crimson Rose)
Nymphalidae
Euploea crameri (Spotted Black Crow)
Total Number of protected species under Schedule I of WPA: 2
Schedule II
Euchrysops cne us (Gram Blue)
Lampides boeticus (Pea Blue)
Mahathala ameria (Falcate Oak Blue)
Lycaenidae
Total Number of protected species under Schedule II of WPA: 3
Schedule IV
Appias libythea (Striped Albatross)
Pieridae
Total Number of protected species under Schedule IV of WPA: 1
30
Mangrove Area
o. of s ecies
25
Reclaimed Area
20
0
15
40
mber of s ecies
60
80
100
Papilionidae
10
5
Pieridae
e
Nymphalidae
VF
F
IF
R
He
sp
eri
ida
ae
nid
Lyc
ae
ae
lid
ha
mp
Pie
rid
ae
Ny
Pa
p
ilio
nid
ae
0
Lycaenidae
i re .
er y families com ared amon man ro e and
reclaimed areas in ndian ndarbans in terms of their s ecies
richness.
rare’ (n 41; 53.9% of the taxa), as they were recorded
only in one or two sites. Most of the rare species
belonged to Lycaenidae and Hesperiidae (Fig. 6).
No butterfly species in the Sundarbans were recorded
as very frequent’ during the study period. A meagre
10.5% of the species (n 8) were found to be frequent’
in both the reclaimed areas and the mangrove forests
(Fig. 6). Hesperiidae and Lycaenidae families remained
devoid of any frequently observed species (Fig. 6).
Three species of Tiger butterflies, viz., Danaus genutia
Cramer, D. chrysippus Linnaeus and Tirumala limniace
Cramer were recorded with FO 60%. Striped Tiger,
Danaus genutia was the only species to occur in most
of the sampled sites (12 out of 18 sites). Besides this,
the deltaic region does not hold any endemic species of
butterflies.
Of the 76 butterfly species in the Indian Sundarbans,
six are legally protected under the Indian Wildlife
(Protection) Act, 1972 (Table 3).
6088
20
Hesperiidae
i re . he cate ories of fre ency of occ rrence
for b er y
families in ndian ndarbans. VF Very Frequent; F Frequent;
IF Infrequent; R Rare. Typical features include absence of Very
Frequent’ class and presence of Rare’ class in maximum, with
lycaenids and hesperiids taking the greatest share.
As compared to an inventory of 73 butterfly species
from the Indian Sundarbans during the present study,
earlier workers like Mandal & Nandi (1989) recorded
eight species only. Species sampled during the field
surveys when compared to that obtained from literature
surveys revealed that three species, viz., Graphium
nomius, Eurema brigitta and Idea agamarschana were
not recorded by the author during his present study.
These three butterflies were reported to occur in both,
the dense and the scanty mangrove forests of the Indian
Sundarbans (Mandal & Nandi 1989). The present article
thus includes these three species (marked with in Table
1) along with the species inventoried to obtain a total of
76 butterfly species in the Indian Sundarbans (Table 1).
Butterfly species recorded in the Indian Sundarbans
were found to be distributed among five families:
Papilionidae, Hesperiidae, Pieridae, Lycaenidae and
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
ndarban ios here eser e
Nymphalidae, under a single superfamily Papilionoidea
(van Nieukerken et al. 2011). Large variations have been
found to exist among the butterflies at both their generic
and specific categories as reflected in Table 2. Such
variations can be attributed to the habitat heterogeneity
and diversity of the range of larval host plants that
have colonized the delta from the mainland over the
years, primarily due to reclaiming of the previously
occurring dense mangrove forest lands for colonization,
agriculture and aquaculture practices.
The tendency of the sample-based species and genus
rarefaction curves, not to reach a plateau with increasing
sample numbers, indicates an addition of some newer
species with further sampling. However, the tendency
of the number of genera to reach an asymptote sooner
than the number of species sampled reflects a pattern
inevitable for any case of category-subcategory sampling
curves (Gotelli & Colwell 2001). Although the genus has
fewer members than the species in the present case, the
respective rarefaction curves are closely placed because
of the occurrence of numerous monospecific genera
(n 44; 77.19% of the taxa) of butterflies in the Indian
Sundarbans. This unique relationship is reflected in the
species-genus ratio curve (Fig. 4) revealing a very gentle
but non-linear positive slope.
The species-genus ratio has long been in practice to
describe community patterns as well as to surmise levels
of competitive interactions among species within genera
(Gotelli & Colwell 2001). A very low species-genus
ratio (S/G 1.33) for the butterflies of the Sundarbans,
following Elton’s (1946) proposition, indicates strong
intra-generic competition, limiting in turn congeneric
coexistence as hypothesized by Darwin (1859).
Widespread observations revealed that species-genus
ratios were usually smaller for island than mainland
communities (Elton 1946; Simberloff 1978). As most
of the Indian Sundarbans delta is comprised of island
complexes, the present findings remain consistent with
the aforementioned propositions.
Interference in intrusion of saline water (resulting
from tidal influx) and reclamation of forest lands for
the ever-growing demand for human settlement and
agricultural practices in the upper and middle estuarine
regions of the Sundarbans has resulted in modifying
the floristic spectrum, indulging the intensification of a
diverse array of larval food plants as well as nectar plants
for adult butterflies. Such a phenomenon facilitated an
immigration of diverse butterflies from inland areas
thereby changing their community composition in the
butterfly-poor mangrove ecosystems and the nonforest areas of the Indian Sundarbans delta. Deficient
ho dh ry
food sources, particularly in terms of non-nutritious
mangrove leaves and stressed environmental conditions,
like strong sunlight, high temperature and desiccation
(Kathiresan & Bingham 2001) may have made their
species richness in such ecosystems correspondingly
low. This is reflected by their fairly high beta diversity
existing between reclaimed and mangrove forested
areas of the Indian Sundarbans. Earlier studies also
indicate fewer species to be restricted to mangroves in
other parts of the world (Corbet & Pendelbury 1992).
Thus, many of the species recorded in the Sundarban
mangrove forests in the present study can be suggested
to be visitors that frequently visit the mangrove flowers
for the rich nectar sources.
Although all butterfly families show a higher species
richness in reclaimed areas, prominent contribution
towards their difference among the reclaimed and
mangrove areas were largely attributed to the lycaenids
and hesperiids (Fig. 5). The underlying reasons for
such an event seem to be twofold: (i) the extreme
deficiency in larval food plants for these two families in
the mangrove forests and (ii) the increased abundance
of various cultivated and decorative plants (Arecaceae,
Combretaceae, Fabaceae, Mimosaceae, Poaceae,
Rhamnaceae, Rubiaceae, Rutaceae, Zinziberacaeae and
others) in reclaimed areas of the Indian Sundarbans that
serve as preferred food plants for their larvae.
Forty one species of butterflies in Sundarbans have
been considered rare in terms of their frequency of
occurrence in the deltaic region. Rare species like the
Spotted Black Crow Euploea crameri Lucas is a resident
of the mangrove belt (Mondal & Maulik 1997), although
it was mentioned to be accidentally introduced by
shipping on a small, flat alluvial island of Sundarbans
(Fruhstorfer 1910). Mangrove Nymph I. agamarshchana
C. & R. Felder is rare in the Indian Sundarbans, from
Soumyajit Chowdhury
er ies of
ma e . he de radin habitat for mall almon Arab olo s a a a
abrici s
at a reclaimed area in annin ndian ndarbans.
he inset sho s a male indi id al of the s ecies.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
ndarban ios here eser e
ho dh ry
a
b
c
d
ma e . i er
innae s
er ies of ndian ndarbans delta a tri ed i er anaus enu a ramer
b Plain i er anaus c r si
c hite i er anaus elani us ramer
d l e i er iru ala li niace ramer
.
where it was first reported by Mondal & Maulik (1997);
although Talbot (1949) mentioned it hitherto as very
common in the mangrove forests of the Bangladesh
Sundarbans. Another rare butterfly–the Small Salmon
Arab Colotis amata Fabricius, being a specialist’ and
monophagous on Azima tetracantha (a semi-saline,
mangrove-associated species belonging to family
Salvadoraceae) was found to be locally threatened
because of habitat modifications and rapid depletion of
the host plant populations (Chowdhury & Soren 2009)
in some reclaimed areas of the Sundarbans (Image 3).
The deltaic environment of the Sundarbans proved
hostile for butterfly abundance as no butterfly species
were found to be very frequent’ (Fig. 6). Only 8 species
were found to be frequent’ in both the reclaimed areas
and mangrove forests , viz., the papilionid Papilio polytes
Soumyajit Chowdhury
er ies of
us
Linnaeus, the pierids Catopsilia pyranthe Linnaeus and
Eurema hecabe Linnaeus, and the nymphalids Euploea
core Cramer, Elymnias hypermenstra Linnaeus, Tirumala
limiace Linnaeus, Danaus chryssipus Linnaeus and D.
genutia Cramer. Hesperiidae and Lycaenidae families
remained devoid of any frequently observed species.
Tiger butterflies (Danaus genutia Cramer, D. chrysippus
Linnaeus, D. melanippus Cramer and Tirumala limniace
Cramer) (Image 4) were found to be unique to the
mangroves. Except for the White Tiger, D. melanippus,
the other three species were found to be more widely
distributed in the Sundarbans. Moreover, by virtue of
the presence of mangrove-associated plant species
belonging to the Asclepiadaceae family: viz., Hoya R.
Br. and Tylophora R. Br. (Banerjee et al. 2002) that are
preferred by the Striped Tiger, D. genutia as their larval
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
er ies of
ndarban ios here eser e
food plants, the latter has been found to be widely
frequent in the mangrove forests of the Sundarbans as
well.
In the butterfly-poor Sundarbans deltaic region
of eastern India, six of the recorded 76 species,
approximately 8% of the butterflies are legally
protected under the Indian Wildlife (Protection) Act,
1972 (Anonymous 1997). Of these, two are Schedule I,
three are Schedule II and one is a Schedule IV species;
the former two gain extreme importance because of
the highest level of legal protection they receive in
the country. Table 3 shows the taxonomic breakdown
of these scheduled butterflies. Among the scheduled
species, the Spotted Black Crow E. crameri has been
recorded only from the deltaic regions of the Sundarbans
and the mangrove and secondary forested regions of
Orissa (Kehimkar 2008). Moreover, legally protected
species like Gram Blue Euchrysops cne us and Pea Blue
Lampides boeticus are considered ‘pests’ for a variety of
crops (NBAII 2013) and many-a-times eradicated in large
numbers (mainly at their larval stages) along with other
insect pests due to the indiscriminate use of pesticides
in different agricultural tracts of the reclaimed areas of
delta.
Habitat modifications and change in local climatic
conditions (Danda et al. 2011), primarily due to human
interventions, are the potent factors for organizing
the butterfly community in the fragile ecosystems
of Sundarbans. Such a change, as initiated in the
otherwise lepidoptera-poor mangrove ecosystems of
Sundarbans may interfere with their normal ecosystem
functioning. Under the present scenario of increased
stress towards these deltaic mangrove ecosystems,
further studies on the exploration of larval food plants
and niche specificities of the unique species (like the
Tiger butterflies, Danaus genutia) in this region may
help, in the long run, to consider them as focal species
(viz., indicator species) for conservation programmes
and ecosystem management of this highly threatened
and bio diverse deltaic region.
A ra ala . . ta A. . Ahmed . mith M. an Aalst
.
Development and Climate Change in Bangladesh: Focus on Coastal
Flooding and the Sundarbans. Report no. COM/ENV/EPOC/DCD/
DAC(2003)3/FINAL. Organisation for Economic Cooperation and
ho dh ry
Development, Paris, 70pp.
Anonymo s
. The ildlife (Protection) Act,
(as amended
upto
) ith rules uptil
. Natraj Publishers, Dehra Dun, 158pp.
aner ee . . .A. ao A. . . hastry
. hosh
. Diversity
of Coastal Plant Communities in India. Kolkata: ENVIS-EMCBTAP,
Botanical Survey of India, Ministry of Environment and Forests,
524pp.
ha dh ri A. . A. ho dh ry
. angroves of the Sundarbans.
olume India. World Conservation Union, Gland, 247pp.
ho dh ry .
. Ecology of Fiddler Crabs in the Sundarban
Mangrove Ecosystems, West Bengal, India. PhD Thesis. School of
Oceanographic Studies, Jadavpur University, 153pp.
ho dh ry .
. oren
. Rediscovery of Small Salmon Arab,
Colotis amata Fabricius from Saline and Semi-Saline Areas of West
Bengal, India. Journal of Threatened Taxa 1(6): 351–352; http://
dx.doi.org/10.11609/JoTT.o2127.351-2
ol ell . .
. EstimateS: Statistical Estimation of Species
ichness and Shared Species from Sample. ersion . . . Freeware
for Windows and Mac OS (Internet). http://viceroy.eeb.uconn.edu/
EstimateS . Downloaded on 8 July 2013.
orbet A. .
.M. Pendleb ry
. The Butterflies of alay
Peninsula, th edition (Revised by J.N. Eliot). Malaysian Nature Society,
Kuala Lampur, 594pp.
anda A.A. . ris anthan A. hosh . andyo adhyay
. a ra
. Indian Sundarbans Delta - A ision. World Wide Fund for
Nature-India, New Delhi, 40pp.
ar in .
. The Origin of Species by eans of Natural Selection.
John Murray, London, 502pp.
lton
.
. Competition and the structure of ecological
communities. Journal of Animal Ecology 15: 54–68; http://dx.doi.
org/10.2307/1625
ans
. .
. The Identi cation of Indian Butterflies. Bombay
Natural History Society, Bombay, 464pp.
r hstorfer .
. Familie Danaidae part , pp. 191–200, 209–216,
225–272. In: Seitz, A. (ed.). Die ross-Schmetterlinge de Erde. Band I .
Die Tagfalter. A. Kernen, Stuttgart, 1197pp.
otelli . .
. . ol ell
. uantifying Biodiversity: procedures
and pi alls in the measurement and comparison of species richness.
Ecology Letters 4: 379–391; http://dx.doi.org/10.1046/j.14610248.2001.00230.x
. . arim M. Asad aman
. Mahtab eds.
.
ulnerability and Adaptation to Climate Change for Bangladesh.
Kluwer Academic Publishers, Dordrecht, The Netherlands, 147pp.
. The I CN ed List of Threatened Species. ersion
. .
http://www.iucnredlist.org . On-line version dated 15 April 2012.
r inen
.
. Species-to-genus ratios in biogeography: a
historical note. Journal of Biogeography 9: 363–370; http://dx.doi.
org/10.2307/2844723
athiresan .
. . in ham
. Biology of mangroves and
mangrove ecosystems. Advances in arine Biology 40: 81–251.
ehim ar .
. Book of Indian Butterflies. Bombay Natural History
Society, Oxford University Press, Mumbai and Delhi, 513pp.
Ma rran A. .
. easuring Biological Diversity. Blackwell Science
Ltd., UK, 256pp.
Mandal A. .
. . andi
. Fauna of Sundarban Mangrove
Ecosystem, est Bengal, India. Fauna of Conservation Areas .
Zoological Survey of India, Kolkata, 116pp.
Mandal . .
. . Ma li
. Insecta: Lepidoptera: Rhopalocera
Papilionidae and Danaine Nymphalidae, pp. 755–793. In: Director,
Zoological Survey of India (ed.). State Fauna Series Fauna of est
Bengal. Part . Zoological Survey of India, Kolkata, 793pp.
as ar . .
. . Mandal
. Ecology and Biodiversity of Indian
angroves. Daya Publishing House, Delhi, 754pp.
A
. Castalius rosimon, Euchrysops cne us and Lampides
boeticus. In: Insects in Indian Agroecosystems. 2013 National Bureau
of Agriculturally Important Insects. http://www.nbaii.res.in/
insectpests/index.php . Downloaded on 02 September 2013.
e
. .
. Launching and steering flagship Lepidoptera for
conservation benefit. Journal of Threatened Taxa 3(6): 1805–1817;
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
er ies of
ndarban ios here eser e
ho dh ry
http://dx.doi.org/10.11609/JoTT.o2621.1805–17
anyal P. . . aner ee M. . ho dh ry
. Dancing Mangals
of Indian Sundarbans. Journal of the Indian Society for Coastal
Agricultural esearch 2(1): 10–16.
e eriano . A. . Mo ra . . . li iera M. . ordeiro Ara o
. . antas
. Micro-phytoplankton richness in Contas River,
state of Bahia, northeastern Brazil. Check List 8(2): 218–223.
imberlo
.
. Use of Rarefaction and Related Methods in
Ecology, pp. 150–165. In: Dickson, K.L., J. Jr. Cairns & R.J. Livingston
(eds.). Biological Data in ater pollution Assessment uantitative
and Statistical Analyses. American Society for Testing and Materials,
Philadelphia, PA, USA.
albot .
. The fauna of British India including Ceylon and Burma
- Butterflies . Taylor and Francis Ltd., London, 506pp.
an ie er en . . . aila . . itchin
.P. irstensen . . ees
. Minet . Mi er M. M tanen . . e ier . . imonsen .
ahlber . . en . ahiri . Adams i . ai eras . artsch
.A. en tsson . . ro n . . cheli . . a is . . Prins . .
Prins M. . stein P. entili Poole . ielis P.
ensch iler A.
a smann . . ollo ay A. allies . arsholt A. . a ahara .
oster M. . o lo . . afontaine . amas . . andry . ee
M. ss . . Par . Pen . ota A. chintlmeister . . chimdt
. . ohn M.A. olis .M. ermann A. . arren . eller . .
a o le
. . olot hin
A.
ic
. Order Lepidoptera
Linnaeus, 1758. In: Zhang, Z.- . (ed.) Animal Biodiversity: An Outline
of Higher-Level Classification and Survey of Taxonomic Richness.
Zootaxa 3148: 212–221.
hi a er . .
. Vegetation of the Siskiyou Mountains, Oregon
and California. Ecological onographs 30: 279–338. http://dx.doi.
org/10.2307/1943563
ynter lyth M.A.
. Butterflies of the Indian egion. Bombay
Natural History Society, Bombay, 523pp.
c ler . . alachandran . . ntin M. anc M. ashi a i
. . a o . . Mahes aran A. harma . . yroech o s i
.
ebb
. The Indian Sunderbans: an important wintering site for
Siberian waders. ader Study roup Bulletin 108: 42–46.
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6082–6092
o rnal of hreatened a a
.threatenedta a.or
ly
P
M
Aboli
l arni Mandar . atar
mesh A asar ar
An radha
ISSN
Online 0974–7907
Print 0974–7893
adhye
MACS- Agharkar Research Institute, Gopal Ganesh Agarkar Road, Pune, Maharashtra 411004, India
aboli.kulkarni5 gmail.com, 2 datarmandar gmail.com (corresponding author), 3 umesh.awasarkar gmail.com,
4
upadhye.anuradha gmail.com
1,2,3,4
P
A
1
Abstract Sacred Groves are the forest patches dedicated to a local
deity, which have been conserved since centuries and play a very
important role in conserving many threatened plants as well as animal
species. In the present work, we have studied fifteen Sacred Groves
in Pune District for their floristic diversity. A total of 296 species were
recorded of which five species show their northernmost distribution to
the Western Ghats in the Sacred Groves of Pune District. The present
paper highlights this unique feature of sacred groves as the abodes of
rare species having their northernmost distribution recorded in the
grove.
ey ords Northernmost records, Pune, Sacred Groves.
India has a rich cultural heritage of dedicating
groves, ponds, and rivers to God. These are the patches
of forests dedicated to a local deity and significant not
only from cultural but also from a conservation point of
view. The religious beliefs prohibit cu ng, or lopping
in sacred groves. Sacred groves exist in various sizes
from a few trees to a few square kilometers. They have
been preserved for the last 2500 years, since the time
agriculture was first introduced in northern Western
Ghats (Gadgil & Vartak 1975). This tradition dates back
to the pre-agricultural, hunting gathering stage of the
society. These groves are abodes of many rare and
endemic plants and animal species. They harbor plants
of various utilities like timber, medicinal plants, NTFP,
etc. Many species of birds, animals and plants might
have become extinct on the nonexistence of these
groves (Gadgil & Vartak 1973).
In India, sacred groves are found almost in all states
with differing numbers and areas. Maharashtra has
2800 sacred groves covering approximately 35,700km2
of area, most of which are located in the Western Ghats
or Konkan and harbor about 800 species of plants
(Deshmukh 1999). Sacred groves are locally known as
Devrai, Devpan, Devrahat, Devara kadu, Kavus, Orans,
Than etc. The Western Ghats of Maharashtra is spread
from river Tapti to Tilari ghat in the south. This stretch
has many vegetation types including evergreen, semievergreen, moist deciduous forests (Champion & Seth
1968) interspersed with rocky outcrops (Watve 2013).
The forest patches in the Western Ghats of Maharashtra
are not continuous as that of the southern Western
Ghats. Many tree species that are common in southern
Western Ghats are rare in northern Western Ghats.
Presently, the forest patches of northern Western Ghats
are confined to protected areas like Bhimashankar,
Koyna, Chandoli and Radhanagari (Gadgil et al. 2011).
Sacred Groves gain immense importance as they
: http://dx.doi.org/10.11609/JoTT.o3644.6093-100
ditor B. Ravi Prasad Rao, Sri Krishnadevaraya University, Anantapur, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3644 | Received 29 May 2013 | Final received 01 July 2014 | Finally accepted 03 July 2014
itation Kulkarni, A., M.N. Datar, U. Awasarkar & A. Upadhye (2014). Northernmost distribution of five tree species to the Western Ghats from the sacred groves
of Pune District, Maharashtra, India. Journal of Threatened Taxa 6(8): 6093–6100; http://dx.doi.org/10.11609/JoTT.o3644.6093-100
o yri ht © Kulkarni et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin Forest Department, Maharashtra State.
om etin nterest The authors declare no competing interests.
Ac no led ements The authors are thankful to Director, Agharkar Research Institutes, Pune and In-charge Botany Group for providing necessary facilities and
encouragements. The authors are also thankful to Forest Department, Maharashtra state for funding this project.
orthernmost distrib tion of
e tree s ecies
Kulkarni et al.
maintain pristine but fragile patches of forest in addition
to these inadequately protected areas. As these forests
are not continuous, the species distribution throughout
northern Western Ghats is also fragmented. There are
many trees species restricted only to the sacred groves.
Sacred groves shelter rare and endemic flora and
fauna and are refugia and breeding grounds of many
animals. They are also abodes of wild relatives of
cultivated plants, repositories of medicinal plants, source
of perennial water, etc. (Ghate et al. 2004). This short
communication highlights an additional significance
of sacred groves as remnants of forest patches which
shelter rare species, as the sacred groves themselves
are the northernmost known locations of five arboreal
species documented during this study.
M
M
Fifteen sacred groves spread across Pune District
were studied for their plant diversity. Surveys were
conducted from March 2012 to September 2013. A
comprehensive checklist of all the plants present in the
groves was prepared. The specimens were identified
using local floras (Cooke 1901–1908; Sharma et al.
1996; Singh & Karthikeyan 2000; Singh et al. 2001).
Plants were collected and processed using conventional
ma e . ocation of the sacred ro es for northernmost records Ma
collection methods (Jain & Rao 1977). Identities
of plants were confirmed by comparing them with
authentic specimens deposited at Agharkar Research
Institute herbarium (AHMA) and Herbarium of Botanical
Survey of India, Western Regional Station, Pune (BSI).
The herbarium specimens collected during the present
work are deposited in AHMA. A total of 296 species of
angiosperms were reported from these sacred groves.
Available literature on plant diversity of northern
Western Ghats were referred to (Santapau 1953, 1958;
Kulkarni 1988; Almeida 1990; Lakshminarasimhan &
Sharma 1991; Deshpande et al. 1993–1995; Kothari &
Moorthy 1994; Pradhan & Singh 1999; Yadav & Sardesai
2002; Patil 2003) for distribution of these species.
During this documentation it was found that five species
show their northernmost distribution to three groves
in Pune District (Image 1). A brief citation, description,
notes on distribution and ecology of these five species in
the Western Ghats are provided herewith.
ros s ac s indica Dalzell in Hooker’s J. Bot. Kew
Gard. Misc.2:41.1850; Hook. f., Fl. Brit. India 5:406.1887;
T. Cooke, Fl. Bombay 2:102. 1967 (Repr.); Airy Shaw in
Kew Bull. 26:210.1972; N. P. Balakr. & Chakrab., Family
as re ared sin
i a
ersion .
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
e tree s ecies
Kulkarni et al.
Euphorb. India 157. 2007. (Euphorbiaceae).
Small trees, up to 5m tall. Leaves alternate, 10–25x4–
8 cm, oblong-lanceolate, apex and base acute, margins
coarsely and sharply serrate with incurved spinulose
teeth, coriaceous. Male flowers minute, solitary in axils
of small imbricating bracts, arranged in axillary or supra
axillary clusters. Female flowers solitary, pedicellate.
Capsules 3-lobed, 1–1.2 cm across, glabrous, red. Seeds
globose, pale brown.
Flowering and Fruiting: October–March.
Distribution: Indo-Malayan Region. Western Ghats
of India. In Maharashtra this species was earlier
reported form Kolhapur & Ratnagiri (Singh et al. 2001).
Dhuprahat (Bhor Taluka of Pune District) forms the
northernmost distribution of the species.
Exsiccata: 28353 & 28630 (AHMA), 07.ii.2013,
Dhuprahat (Images 2–4).
Occurrence: Very rare in thick evergreen forests.
anariu
s ric u
Roxb., Fl. Ind. Ed. Carey
3:138.1832; A.W. Benn. in Hook. f., Fl. Brit. India 1:534.
1875; T. Cooke, Fl. Bombay 1:214.1967(Repr.); Chithra
ma e
.
erbari m of
ros s ac s indica
Mandar Datar
orthernmost distrib tion of
ma e .
ros s ac s indica male o ers
& A.N. Henry in Hajra et al., Fl. India 4: 440. 1997.
(Burseraceae).
Trees up to 20m tall; branches velvety tomentose.
Leaves imparipinnate, 5–10x2–4 cm, elliptic or
oblanceolate, acuminate at apex, obtuse or subcordate
A MA
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
e tree s ecies
Kulkarni et al.
Mandar Datar
orthernmost distrib tion of
s ric u
trees on the bac ro nd of
Mandar Datar
ma e . o anariu
h rahat
ma e . anariu
s ric u
tree
at base, margins serrate or crenate, rusty villous below,
young leaves reddish. Inflorescence of axillary panicles.
Flowers pale yellow or white, 3-merous, c. 8mm across;
calyx tube campanulate, pubescent; stamens 6, free
from disc. Drupes c. 3.7–5 cm long, ellipsoid or ovoid,
tapering at both ends, stony, hard, bony.
Flowering and Fruiting: February–May.
Distribution: Western Ghats and Myanmar. In
Maharashtra the species is distributed in Kolhapur, Pune
and Raigad districts (Singh & Karthikeyan 2000). The
Dhuprahat Sacred Grove (Bhor Taluka of Pune District)
is its northernmost known location in northern Western
Ghats.
Exsiccata: 17364 (AHMA), 18.iv.1987, Dhuprahat
(Images 5–7).
Occurrence: Rare in thick evergreen forests.
6096
ma e . erbari m of anariu
s ric u
A MA
u rasia a ularis ar. elu na (M. Roem.) King in
J. Asiat. Soc. Bengal. 64:88.1895. Hiern in Hook. f., Fl.
Brit. India 1:568.1875 (as Chickrassia’); T. Cooke, Fl.
Bombay 1:230.1967 (Repr.); S.S. Jain & Bennet in Hajra
et al., Fl. India 4:482.1997. (Meliaceae).
Tall trees, 5–8 m tall; bark dark greyish, lenticellate.
Leaves alternate, abruptly pinnate; leaflets 5–12 pairs,
3–10 x 2–4 cm, ovate or oblong, apex acute or acuminate,
base inequilateral, tomentose above, velvety beneath.
Inflorescence of terminal panicles, shorter than leaves.
Flowers dirty white; calyx short, 5-toothed; petals linearoblong; staminal tube cylindric, glabrous, with 10 short
teeth. Capsules 4–6 x 2.5 cm, ovoid, 3- valved, spli ng
at tips during dehiscence. Seeds numerous, flat, closely
packed, broadly winged.
Flowering and Fruiting: February–September.
Distribution: Andaman and Nicobar Islands, Western
Ghats of Karnataka and Maharashtra, Sri Lanka and
Myanmar. In Maharashtra this variety is distributed
in Pune, Satara, Raigad and Sindhudurg districts
(Singh & Karthikeyan 2000). In Pune District it was
earlier reported from Khandala (Santapau 1953). The
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
orthernmost distrib tion of
e tree s ecies
Kulkarni et al.
Mandar Datar
Ahupe SG (Images 8–10)
Occurrence: In patches of semi-evergreen forest.
ma e .
u rasia a ularia ar. elu na tree inset fr it
present distribution of this species from Ahupe Sacred
Grove (Ambegaon taluka of Pune District) forms the
northernmost record in the Western Ghats.
Exsiccata: 28356 & 28653 (AHMA), 14.iii.2013,
ma e
.
u rasia a ularia ar. elu na
ris ca dac loides Gaertn., Fruct. 1: 195, t. 41, f.
2a-d. 1788; M. beddomei King in Ann. Roy. Bot. Gard.
Calcutta 3: 291, t. 118, f. 1-18. 1891. M. laurifolia Bedd.,
Fl. Sylv. t. 267. 1872; Hook. f., Fl. Brit. India 5: 103. 1886.
(Myristicaceae).
Trees, up to 20m high; branchlets glabrous. Leaves
12.5–22 x 5.5–9 cm, elliptic or oblanceolate, bluntly
acute at apex, rounded at base, coriaceous, glaucous
beneath. Flowers cream-coloured; male flowers in
pedunculate cymes; perianth ovoid, rusty tomentose;
female flowers few-flowered, in axillary cymes; perianth
cuneate. Drupes c. 6.5x3.8 cm, broadly ovoid, yellowtomentose. Seeds with red aril.
Flowering and Fruiting: October–May.
Distribution: Western Ghats and Sri Lanka. In
Maharashtra this species is reported from Raigad,
Satara, Sindhudurg (Singh et al. 2001). Durgawadi
A MA
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
e tree s ecies
Kulkarni et al.
Agharkar Institute
orthernmost distrib tion of
ma e
.
ma e
. erbari m of
ris ca dac loides
ris ca dac loides
a ernae on ana al erni olia L., Sp., Pl. 211.1753;
Nicolson et al. in Regnum Veg. 119 (Interpr. Rheede’s
Hort. Malab.):57.1988. Ervatamia alternifolia (L.) S. M.
Almeida, Fl Savantwadi 1:251.1990. Tabernaemontana
heyneana Wall. in Edwards’s Bot. Reg. 15: t. 1273.1829;
Hook. f., Fl. Brit. India 3:646.1882; Karthik. et al., Fl. Pl.
India-Dicot. 1:138.2009. Ervatamia heyneana (Wall.)
T. Cooke, Fl. Bombay 2:134.1904 2:196.1967(Repr.)
(Apocynaceae).
Large shrubs or small trees, up to 5m tall. Leaves 10–
20 x 3.5–5.5 cm, oblong-lanceolate or elliptic-lanceolate,
apex shortly acuminate, base acute, glabrous; main
nerves 12–16 pairs. Inflorescence in many flowered
cymes. Calyx c. 5mm long, lobes 2, rounded at apex;
corolla tube 1.5–2.5 cm long, inflated, lobes crisped,
boat shaped. Follicles 2.5 – 3 x 1 – 1.2 cm, orange when
ripe, curved. Seeds 8–10 mm long, surrounded by red
pulp.
Flowering and Fruiting: February–September.
Distribution: Endemic to western and southern
India (Andhra Pradesh, Goa, Gujarat, Karnataka, Kerala,
Maharashtra and Tamil Nadu). In northern Western
Ghats the species is distributed upto Satara District (Singh
et al. 2001), but not reported in Khandala (Sanatapau
1953). In Konkan region the northernmost record of the
species is Raigad District (Kothari & Moorthy 1993). In
the present work the species is reported from Ahupe
Sacred Grove (Ambegaon Taluka of Pune Distict) which
forms its northernmost record.
Exsiccata: 28357 (AHMA), 14.iii.2013, Ahupe (Images
13–14).
Occurrence: Common in moist deciduous forests.
A MA
Mandar Datar
(Junnar Taluka of Pune District) forms its northernmost
record.
Exsiccata: 28354 (AHMA), 04.iii.2013, Durgawadi
(Images 11–12).
Occurrence: Common in semi-evergreen forests.
ma e
6098
. a ernae on ana e neana o er
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
orthernmost distrib tion of
e tree s ecies
Kulkarni et al.
Western Ghats of Maharashtra, recorded from these
sacred patches of forests.
Sacred Groves are relics of the primary forests of the
Western Ghats and are centers of endemism for both
plants and animals. A casual approach to managing
these forest fragments has led to the destruction of
these forest fragments. Weakening of religious beliefs
is one more concern for sacred groves. In regions like
northern Western Ghats, where there is a limited scope
of declaring new protected areas due to social, economic
and political constraints, protection of the sacred grove
is of immense importance.
ma e
. a ernae on ana e neana
A MA
Due to a rapid growth of human population and
increased urbanization, forest fragments in the tropics
are under severe threat. Anthropogenic pressures
on forests have resulted in severe degradation of the
ecosystems and have a serious impact on the biodiversity
of the region. They not only reduce the biodiversity of
the region but change the species composition of the
region over time. Wagh & Ghate (2002) report loss of
60% of fish fauna from Mula-Mutha in the past 60 years.
Recently, Kulkarni & Subramanian (2013) reported
a loss of 31% odonate fauna from the region. Loss of
species at such a rate is alarming and demand dedicated
efforts for conservation of this natural wealth. Sacred
Groves, that have conserved these forests for ages, are
also subjected to this impact. The northern Western
Ghats in Maharashtra have patchy distribution of many
tree species due to a lack of continuous forest patches.
Sacred groves provide suitable habitats to many such
species. The present work reports the northernmost
distribution of five species of angiosperms to the
Almeida .M.
. The Flora of Sawantwadi, Maharashtra, India.
Journal of Economic & Taxonomic Botany, Additional Series 2
Volumes. Scientific Publishers, Jodhpur, 411 304pp.
ham ion . .
. . eth
. A Revised Survey of The Forest Type
of India. Manager of Publications, New Delhi, 404pp.
oo e .
. Flora of Presidency of Bombay - 2 Volumes.
Taylor & Francis, London 645 1083pp.
eshm h . .
. Final Report of the World Bank aided Maharashtra
Forestry Project Conservation and Development of Sacred groves in
Maharashtra submitted to the Department of Forest, Government of
Maharashtra. Bombay Natural History Society, Mumbai, 289pp.
esh ande . . . harma
M.P. ayar
. Flora of
Mahabaleshwar and Adjoining, Maharashtra. Volume 1 - 1993 &
Volume 2 - 1995. Botanical Survey of India, Calcutta, 776pp.
ad il M.
. . arta
. Groves dedicated to the gods. The
Illustrated Weekly of India, 4pp.
ad il M.
. . arta
. Sacred groves of India: A plea for
continued conservation. Journal of the Bombay Natural History
Society 72: 314–320.
ad il M. . . rishnan . . aneshaiah . . i ayan . or es .
mar . oronha . aya
. . bramanian . . arma .P.
a tam . . a al nd
. . brahmanyam
. Report of
the Western Ghats Ecology Expert Panel. The Ministry of Environment
and Forest, Government of India, 522pp.
hate . . . . ane
. . anade ed.
. Focus on Sacred
Groves and Ethnobotany. Prism Publications, Mumbai, xiv 253pp.
ain . .
. . ao
. A Handbook of Field and Herbarium Methods.
Today & Tomorrow’s Printers & Publishers, New Delhi, 157pp.
othari M. .
. Moorthy
. Flora of Raigad District, Maharashtra
State. Botanical Survey of India, Kolkata, 581pp.
l arni A. .
.A.
bramanian
. Habitat and seasonal
distribution of Odonata (Insecta) of Mula and Mutha river basins,
Maharashtra, India. Journal of Threatened Taxa 5(7): 4084–4095;
http://dx.doi.org/10.11609/JoTT.o3253.4084-95
l arni . .
. Flora of Sindhudurg. Botanical Survey of India,
Calcutta, xx 605pp.
a sminarsimhan P.
. . harma
. Flora of Nashik District.
Botanical Survey of India, Calcutta, 644pp.
Patil .A.
. Flora of Dhule and Nandurbar Districts, Maharashtra.
Bishen Singh Mahendra Pal Singh, Dehradun, 700pp.
Pradhan . .
.P. in h
. Flora of Ahmednagar District,
Maharashtra. Bishen Singh Mahendra Pal Singh, Dehradun.
xxvi 707pp.
anta a
.
. The Flora of Khandala on the Western Ghats of
India. Records of Botanical Survey of India, xxvii 396pp.
anta a
.
. The Flora of Purandar or an Enumeration of All the
Phanerogamic Plants Discovered in Purandar during the Year 1944–
1956. New Delhi, 158pp.
harma . . . arthi eyan
.P. in h eds.
. Flora of
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
6099
orthernmost distrib tion of
e tree s ecies
Kulkarni et al.
Maharashtra State. Monocotyledones. Botanical Survey of India.
vi 794pp.
in h .P.
. arthi eyan eds.
. Flora of Maharashtra State.
Dicotyledonous - Vol. 1. Botanical Survey of India, Calcutta, 898pp
in h .P. P. a shminarasimhan . arthi eyan
P. . Prasanna
eds.
. Flora of Maharashtra State. Dicotyledones - Vol II.
Botanical Survey of India, Calcutta, 1080pp.
a h . .
. . hate
. Freshwater fish fauna of rivers Mula
and Mutha, Pune, Maharashtra. Zoos’ Print Journal 18(1): 977–981;
http://dx.doi.org/10.11609/JoTT.ZPJ.18.1.977-81
at e A.
. Status review of Rocky plateaus in the northern
Western Ghats and Konkan region of Maharashtra, India with
recommendations for conservation and management. Journal of
Threatened Taxa 5(5): 3935–3962; http://dx.doi.org/doi:10.11609/
JoTT.o3372.3935-62
ada . . M.M. ardesai
. Flora of Kolhapur District. Shivaji
University, Kolhapur, xiv 680pp.
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6093–6100
o rnal of hreatened a a
.threatenedta a.or
. . a at
Department of Biological Sciences, CBSH, G.B.Pant University of
Agriculture & Technology, Pantnagar, Uttarakhand 263145, India
drds rawat yahoo.com
Uttarakhand is one of the Himalayan states in India
and its area is about 53,483km2, mainly made up of
mountainous terrain. The state hardly covers 1.69%
of the land area of India but hosts more than 27.96%
flowering plant diversity (Karthikeyan 2000; Uniyal et al.
2007) which speaks of the richness of flora here. This
area has been a focus of plant collections as far back as
1796 when Thomas Hardwicke collected plants from the
Alaknanda Valley of Garhwal. Since then, a large number
of plant collectors have explored the area and a great
deal of information was available about the flowering
plants of this area by the beginning of the 21st century.
Based on these collections, floristic reports and their
own collections, Uniyal et al. (2007) compiled a checklist
of flowering plants of Uttarakhand as a baseline data for
writing the flora of Uttarakhand. This valuable document
indicates the presence of nearly 4,700 species of flowering
plants (including 32 species of Gymnosperms and a few
cultivated species).
In routine botanical explorations in different parts of
Uttarakhand a few interesting specimens were collected
and identified with the help of relevant taxonomic
literature and by comparing them with authentic
specimens housed at the herbaria of Botanical Survey
of India (BSI) and Forest Research Institute (DD) at
Dehradun. These species proved as additions to the flora
ly
of Uttarakhand as these were not
mentioned in Uniyal et al. (2007).
Considering it, these species are
being reported here for the first time
ISSN
Online 0974–7907
from Uttarakhand. Their description
Print 0974–7893
including correct name, basionym
P A
(based on The Plant List 2010,
International Plant Name Index 2012
or other recent literature), name in Flora of British India
(Hooker 1872–97), and photographs of their natural state
and their flowering/ fruiting times are provided here in
this communication for future reference and further
correct identification. The species are arranged in the
sequence of families as per Uniyal et al. (2007).
Plant specimens processed following standard
taxonomic procedures (Rao & Sharma 1990) are
deposited and being maintained at G.B. Pant University
Herbarium, Department of Biological Sciences, CBSH
Pantnagar, Uttarakhand, India (GBPUH).
. Tiliacora acuminata (Lam.) Miers in Ann. Mag.
Nat. Hist. ser. 2, 7(37): 39. 1851; Pramanik, Flora of India
1:343. 1993. Menispermum acuminatum Lam., Encycl.
4:101. 1797. T.racemosa Colebr. in Trans. Linn. Soc. Lond.
13: 67. 1822; Hook.f. et Thomson, Fl. Brit. India 1: 99.
1872. (Menispermaceae).
Specimen examined: GBPUH 603/30.1.2013,
21.ix.2011, Haldi near Pantnagar, Uttarakhand, coll. D.S.
Rawat (Images 1,2)
Large, woody stemmed evergreen climbers. Stem
striate, glabrous, young branches pubescent. Leaves
petiolate, petiole up to 4cm, often curved at upper end,
lamina ovate to elliptic-lanceolate, cordate, rounded
or truncate at base, entire, apex rounded, acute or
acuminate, 7–20x6–11 cm, glabrous, first two pairs
of lateral nerves close to lamina base, nerves free at
: http://dx.doi.org/10.11609/JoTT.o3510.6101-7
ditor K.S. Negi, National Bureau of Plant Genetic Resources (NBPGR-ICAR), Nainital, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3510 | Received 01 February 2013 | Final received 13 June 2014 | Finally accepted 22 June 2014
itation Rawat, D.S. (2014). New additions to the flora of Uttarakhand, India. Journal of Threatened Taxa 6(8): 6101–6107; http://dx.doi.org/10.11609/JoTT.
o3510.6101-7
o yri ht © Rawat 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and
distribution by providing adequate credit to the authors and the source of publication.
ndin Self funded.
om etin nterest None.
Ac no led ements The author is grateful to Head, Department of Biological Sciences, CBSH and Dean CBSH, GBPUA & T Pantnagar for providing necessary facilities.
6101
ara hand
a at
D.S. Rawat
Additions to the ora of
ma e . Tiliacora acuminata am. Miers
outer sepals smaller, triangular to ovate, hairy on abaxial
side, innermost three largest, elliptic to ovate, acute
or rounded, glabrous, 5x2.5 mm, erect, upper curved
outward, yellow. Petals 6, free, in one whorl, obovate,
concave, 1–1.5 mm, glabrous, emarginated. In male
flowers stamens 6, antipetalous, longer than petals but
shorter to inner sepals, up to 4.5mm long, filament thick,
anthers small, opening lengthwise, carpellodes few (1–2),
on 1mm long gynophores, or absent. Female flowers with
up to 8 carpels, on gynophores, 2mm long, glabrous, one
ovuled, style curved outward, subapical, as long as ovary.
Fruit a drupecetum, drupes obovoid, subcompressed,
shining red, glabrous, 8–12x6–10 mm, stylar scar subbasal,
embryo curved. In forests and along road sides, climbing
over smaller trees up to 6–7 m.
Flowering and Fruiting: June–October
This species is previously known to occur in Uttar
Pradesh, Bihar, West Bengal, Orissa, Tamil Nadu and
Kerala in India (Sharma et al. 1993) and now being
reported for the first time from Uttarakhand.
. Dunbaria glandulosa (Dalz.) Prain, J. Asiat. Soc.
Bengal 66: 433. 1897; Sanjappa, Leg. India 169. 1992.
Cajanus glandulosus Dalz. In Dalz. & Gibs., Bombay Fl.
73. 1861. Atylosia glandulosa Dalz., J. Linn. Soc. 13: 185.
1873. Atylosia rostrata Baker in Hook.f., Fl. Brit. India 2:
216. 1876. (Fabaceae).
Specimen examined: GBPUH 604/30.1.2013,
30.vii.2012, near Tata Motors, along road, Pantnagar,
Uttarakhand, coll. D.S. Rawat (Images 3,4).
Large vines, up to 5m long, copiously branched. Stem
with glandular and simple hairs, with many obscure
lines of hairs in intermodal region on branches. Leaves
estipulate, petiole up to 14cm, densely hairy, exceeding
base of lateral leaflet joints by up to 3.5cm, petiolule 3–5
margin. Inflorescence axillary raceme like panicles or of
a few flowered pedunculate cymes in raceme, borne on
younger branches, 5–15 cm long, peduncle tomentulose.
Flowers sessile, yellow, 3–4 mm across. Sepals 9, in three
whorls of three each, or one missing in outermost, six
D.S. Rawat
ma e . erbari m s ecimen of Tiliacora acuminata am. Miers
ma e . Dunbaria glandulosa
al . Prain
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
Additions to the ora of
ara hand
a at
covered with long bulbous based golden hairs, style long,
slender, incurved, upper half glabrous, stigma glabrous.
Fruit 5–6.5x0.7–0.9 cm, linear-oblong, acuminate,
blackish-brown, with indistinctly depressed lines between
seeds, densely covered with bulbous based long hairs,
6–8 seeded. Seeds brown, mottled with black, rounded,
compressed, hilum black, of circumference.
Flowering and Fruiting: September–November.
Along road sides, inside forest climbing over shrubs.
This species was known from the Western Ghats,
central India, Assam and West Bengal in India (Sanjappa
1992). Here it is being reported for the first time from
Uttarakhand.
al . Prain
mm; leaves pinnately trifoliate, lateral obliquely rounded,
rounded at base, apex acute, 5-nerved at base of which two
outermost very close to margins, mid nerve closer to upper
margin, adpressed hairy, mainly on nerves on either side,
densely hairy on margins, terminal leaflet rhombic with
rounded lateral angles, equilateral, 5-nerved, outermost
two marginal or submarginal, base rounded, apex acute,
appressed hairy, mainly on nerves on either side, densely
hairy on margins, with numerous brown glands abaxially.
Racemes axillary, few (up to 16) flowered, exceeding leaf,
peduncle with scattered bulbous based long hairs, flowers
solitary or paired at nodes. Flowers ebracteate, pedicel
up to 1.5cm. Calyx tube campanulate, densely covered
with bulbous based hairs, 5-lobed, lobes densely hairy
on margins, lowermost longest, acuminate, rest smaller,
triangular, acute. Corolla three times or more longer than
calyx, standard largest, 2.2x3.0 cm, orbicular, auricled and
clawed; wing 2.2x1.0 cm, auricled and clawed, elliptic to
obovate; keel 2.0x1.3 cm, half rounded, beaked. Stamen
(9) 1, vexillary free, unequal. Ovary base covered with
yellowish nectar ring, ovary sessile, orange, densely
D.S. Rawat
ma e . erbari m s ecimen of Dunbaria glandulosa
. Flemingia lineata (L.) Roxb. ex Ait.f., Hort. Kew ed.2,
4:350. 1812; Baker in Hook.f., Fl. Brit. India 2: 228. 1876;
Sanjappa, Leg. India 176. 1992. Hedysarum lineatum L.,
Sp. Pl. 1054. 1753. (Fabaceae).
Specimen examined: GBPUH 605/30.1.2013,
11.iv.2009, near Petrol Pump, Pantnagar, Uttarakhand,
coll. D.S. Rawat (Images 5,6).
Small shrub, up to 60cm tall. Stem copiously branched,
branches appressed hairy. Stipules leaf opposed,
lanceolate, entire or bifid with long hairy acumen at
apices, persistent, striate, up to 1cm long, brown.
Leaves digitately trifoliate, petiole hairy, up to 4cm long,
petiolules equal, densely hairy; leaflets thinly leathery,
3-nerved at base, nerves raised on abaxial side, central
leaflet obovate to oblanceolate, dorsally glabrescent
at maturity, with numerous, minute, white glands on
abaxial side, cuneate at base, apex acute, 2–6x0.8–2.0
cm; lateral leaflets smaller, obliquely elliptic-lanceolate.
Inflorescence axillary, up to 5cm long panicle, peduncle
glandular hairy. Bracts linear, glandular hairy, up to 2mm
long. Flowers 5–7 mm, pedicel slender, 1–3 mm, glandular
ma e . Flemingia lineata .
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
o b. e Ait.f
Additions to the ora of
ara hand
a at
Specimen examined: GBPUH 442/20.12.2009,
10.Ix.2001, Vasuki Tal area Kedarnath, Uttarakhand, coll.
D.S. Rawat (Image 7).
Loose cushion forming perennial herbs, 8–15 mm
tall, branched at ground level, cushions up to 5cm across.
Proximal axillary shoots leafy, prostrate, with small, distal
leaf rosettes. Leaves in rosette, sessile, linear to oblonglanceolate, entire, glabrous or with sparse eglandular
hairs on dorsal side, acute or obtuse at apex, 1.5–
2.5 0.5–1 mm; leaves on prostrate stems sessile, linear
to oblong-lanceolate, margin entire, glabrous or rarely
with eglandular hairs, apex acute or obtuse, 1.5–2 0.5
mm, somewhat fleshy. Flowering stem terminal, leafless,
ebracteate with solitary flower; pedicel 3 6 mm long,
brown glandular hairy. Flowers minute, 2–3 mm across,
greenish. Sepals 5, erect, 1–1.2 0.6–1 mm, reddish at
apex, ovate to oblong, acute or subacute, adaxial surface
and margins glabrous, or rarely with a few brown-glandular
hairs; veins 3, obscure. Petals absent. Stamens 5, or 6
(rarely), opposite to sepals, equal to sepals, filaments
linear, ca. 0.7mm, anthers 0.3mm, yellow, thecae parallel
on dehiscence. Ovary semi-inferior at anthesis, ovoid to
oblong, to 2.5mm long in fruit; carpels tapered to short
conical styles, styles 0.2–0.3 mm long, exceeding sepals,
stigmas capitate. Seeds spherical to ovoid, shining brown,
ma e . erbari m s ecimen of Flemingia lineata .
Ait.f.
o b. e
hairy. Calyx pubescent, 5-lobed, lobes linear, longer than
tube, lowermost longest. Corolla little exceeding calyx;
standard 5–6x2.5–4 mm, clawed, rounded with two
auricles; wing oblong, auricled, clawed, apex acute; keel
falcate, clawed, apex acute. Stamens (9) 1, vexillary free,
equal. Carpel densely hairy, style long, slender, glabrous
in upper half, stigma capitates, glabrous. Fruit oblongovate, 8–12x6–8 mm, glandular pubescent, yellowishbrown. Seeds 2, black, rounded, 2–2.5x2–2.5 mm, little
compressed, hilum small, white.
Flowering and Fruiting: December–Fabruary.
Along road sides in moist areas in Pantnagar.
Sanjappa (1992) has mentioned the occurrence of
Flemingia lineata from throughout India southward of
Himalaya but Uniyal et al. (2007) lacking specimens have
not mentioned it from Uttarakhand. Thus, it is the first
report of its occurrence in Uttarakhand.
. a i ra a inu ssi a D.S. Rawat, Gornall et al. in
Edinb. J. Bot. 69(2): 211–217. 20012. (Saxifragaceae).
ma e . erbari m s ecimen of a i ra a
inu ssi a . . a at
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
Additions to the ora of
ara hand
a at
smooth, 0.3–0.4 mm.
Flowering and Fruiting: July–October.
On steep slopes in high alpine zones with mosses.
This alpine plant species is a newly described species
from Uttarakhand (Gornall et al. 2012) and considered, till
date, an endemic to Kedarnath area in Uttarakhand.
D.S. Rawat
. Oxystelma esculentum (L.f.) Sm. in Rees, Cycl. 1. 25.
1813; Hook.f., Fl. Brit. India 4: 17. 1893; Karthikeyan et al.,
Fl. Pl. India 1: 177. 2009. Periploca esculenta L.f., Supl. Pl.
168. 1782. (Asclepiadaceae).
Specimen examined: GBPUH 606/30.1.2013,
16.ix.2012, at University boundary, Masjid Colony,
Pantnagar, Uttarakhand, coll. D.S. Rawat (Images 8,9).
Twinning herbaceous climbers, up to 5m long.
Stem glabrous except the younger parts, green. Leaves
opposite, petiolate, petiole up to 2cm, pubescent, blade
linear-lanceolate, entire, acuminate, cuneate or rounded
at base, 5–15x0.5–2.0 cm, pilose on either surfaces,
marginal veins distinct. Inflorescence axillary 1-few (5)
flowered raceme or subumbellate cyme, as long as or
longer than subtending leaf; peduncle pilose. Pedicel
slender, up to 2.5cm long, pubescent. Flower drooping,
1.5–2.5 cm across. Sepal ovate-lanceolate, 1x3 mm,
divided to base, hairy outside. Corolla white, purple
veined on inner side, veins ending before corolla lobe
apices, 5-lobed, lobes triangular, densely white hairy on
the margins, corona densely pubescent at base, hoods
linear, erect, glabrous, incurved at apex, white. Pollinia
up to 2mm long, with dark brown carpusculum. Ovary
glabrous. Fruit elliptic-ovate, inflated, 6x2 cm, glabrous,
rounded at apex.
Flowering and Fruiting: September–December.
Along streams, climbing over grasses, shrubs.
ma e . Oxystelma esculentum .f.
m.
ma e . erbari m s ecimen of Oxystelma esculentum .f.
m.
Oxystelma esculentum is considered as a widespread
species and Karthikeyan et al. (2009) have reported it from
throughout the plains and lower hills of India. However, it
is being reported for the first time from Uttarakhand as it
is not mentioned in Uniyal et al. (2007).
. Hygrophila ringens (L.) R. Br. ex Steud., Nomencl.
Bot. ed.1. 1:418. 1821; Karthikeyan et al., Fl. Pl. India 1:
22. 2009. Ruellia ringens L., Sp.Pl. 635. 1753. Hygrophila
salicifolia (Vahl) Nees, C.B.Clarke in Hook.f., Fl. Brit. India
4: 407. 1884. (Acanthaceae).
Specimen examined: GBPUH 607/30.1.2013,
4.xii.2012, near CIMAP Station in marshy area, Nagla,
Pantnagar, Uttarakhand, coll. D.S. Rawat (Images 10,11).
Marshy herbs, perennial, up to 70cm tall, copiously
branched. Stem decumbent at base, ultimately erect,
rectangular, sparsely pubescent, hairy at nodes. Leaves
opposite, elliptic to lanceolate, blade gradually narrowed
to petiole, appressed hairy on both surfaces, more so on
margins and nerves, entire, obtuse or rounded. Flowers
few (2–6) in axillary clusters, sessile; bracteoles ellipticovate, up to 5mm long,densely long hairy on margins.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
ara hand
a at
D.S. Rawat
Additions to the ora of
ma e
. Hygrophila ringens .
. r. e
te d.
Calyx up to 1.2cm long, lobes linear-lanceolate, shorter
than calyx tube, sparsely hairy outside, densely so on
margins, pubescent inside. Corolla purplish, up to 2.5cm
long, tube to 1.2cm, exceeding calyx, biliped, upper lip
shallowly 2-lobed, lower 3-lobed, lobes ovate, rounded,
hairy inside below lobes. Stamen 4, filaments glabrous,
anther cells equal, parallel, 2mm long, posterior pair of
stamens shorter. Ovary glabrous, style slender, up to
1cm, pubescent in lower half only, stigma 2-lobed, one
much enlarged, other minute. Fruit oblong to lanceolate
capsule, up to 2cm long, 2–3 mm broad, glabrous, 12–16
seeded. Seeds compressed, ovate-rounded, up to 1.5mm
diameter, pubescent on margins.
Flowering and Fruiting: September–January.
In marshy areas making dense thickets.
Karthikeyan et al. (2009) have mentioned its
distribution throughout India but it is not included in
Uniyal et al (2007) which makes it a new addition to the
flora of Uttarakhand.
. Alpinia nigra (Gaertn.) Burtt in Notes Roy. Bot. Gard.
Edinb. 35: 213. 1977; Karthikeyan et al., Florae Indicae
Enumer. Monocot. 289. 1989. Alpinia allughas (Retz.)
Rosc., Baker in Hook.f. Fl. Brit. India 6: 253. 1892. Zingiber
nigrum Gaertn., Fruct. 1: 35. t.12. 1788. (Zingiberaceae).
Specimen examined: GBPUH 608/30.1.2013,
15.viii.2010, Along a stream near Tata Motors, Pantnagar,
Uttarakhand, coll. D.S. Rawat (Images 12,13).
Perennial, rhizomatous, aromatic herbs. Pseudostems
up to 3m tall. Leaves sessile to petiolate, linear-lanceolate
to elliptic-lanceolate, 15–62x2–12 cm, ligule orbicular,
up to 1cm large, hirsute on abaxial side, blade glabrous
except sparse pubescence on the margin of upper half and
apex, acute or acuminate, petiole (in lower leaves) hirsute
6106
ma e
te d.
. erbari m s ecimen of Hygrophila ringens .
. r. e
on adaxial side. Inflorescence terminal, erect, up to 50cm
long panicle, with a few 2–11 cm long branches producing
cincinni of a few (2–6) flowers, peduncle and pedicels
tomentose. Flowers pedicellate, pedicels 0.4–1.5 cm,
tomentose. Calyx tubular, 1–1.2 cm, scarcely 2–3 lobed,
pinkish, pubescent outside, with 1–2 distinct aumens in
bus, persistent. Corolla tube 1–1.2 cm, 3-lobed, pink, lobes
oblong, upto 1.2 cm, pubescent outside, posterior median
lobe outside in bud, cuccullate, with distinct horn in bud.
Labellum obovate,up to 1.5cm long, longer than corolla
lobes, 2-lobed at apex, lateral staminodes subulate, fertile
stamen one, filament robust, 1.0cm, anther lobes 0.7cm,
parallel, curved (straight in fresh specimens), connective
apex with two projecting lobes. Ovary densely pubescent,
rounded, style slender, in stamina cleft, stigma far
exceeding anthers. Capsule globose, blackish, densely
hirsute hairy at maturity, 1.5–2.0 cm, with persistent calyx
funnel. Seed many, 5–6 mm diam.
Flowering and Fruiting: July–October.
Along a perennial stream in one locality.
This species is previously known to occur in the
Himalaya and Eastern India (Karthikeyan et al. 1989),
however, Uniyal et al. (2007) have not reported it from
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
ara hand
a at
D.S. Rawat
Additions to the ora of
ma e
. Alpinia nigra
aertn.
r
Uttarakhand.
All these seven species described above are an addition
to the flora of Uttarakhand, and their specimens collected
from Uttarakhand State do not exist at the Herbarium of
Botanical Survey of India Dehradun (BSD) and Herbarium
of Forest Research Institute Dehradun (DD), indicating
their rare nature. It is hoped that, this information will
prove helpful in their further collection and identification,
and when added with Uniyal et al. (2007), will make the
forthcoming Flora of Uttarakhand more complete and
inclusive.
eferences
AP
. An update of the Angiosperm Phylogeny Group
classification for the orders and families of flowering plants: APG III.
Botanical Journal of the Linnean Society 161: 105-121.
ornall . . . . a at
. han
. Saxifraga minutissima, a
new species from the Garhwal Himalaya, India, and its implications
for the taxonomy of the genus Saxifraga (Saxifragaceae). Edinburgh
Journal of Botany 69: 211–217.
oo er . .
. Flora of the British India ol I- I. London.
arthi eyan .
. A statistical analysis of flowering plants of India.
pp. 201–217. In: Singh, N.P., D.K. Singh, P.K. Hajra & B.D. Sharma
(eds.). Flora of India Introductory olume Part-II, B.S.I. New Delhi,
469pp.
A
endi
ma e
. erbari m s ecimen of Alpinia nigra
r
arthi eyan . M. an a a
. M rthy
. Flowering Plants of
India. Botanical Survey of India, Kolkata, 365pp.
arthi eyan . . . ain M.P. ayar M. an a a
. Florae
Indicae Enumeratio onocotyledoneae, Pune, 435pp.
ao . .
. . harma
. A anual for Herbarium Collections.
B.S.I. Calcutta, 21pp.
an a a M.
. Legumes of India. Bishen Singh Mahendra Pal
Singh, Dehradun, 338pp.
harma . . .P. ala rishnan . . ao P. . a ra
. Flora of
India ol anunculaceae- Barclayaceae. B.S.I. Calcutta, 467pp.
nternational Plant ame nde
. Published on the Internet
http://www.ipni.org accessed 28 January 2013 .
The Plant List
. Version 1. Published on the Internet; http://www.
theplantlist.org/ accessed 28 January 2013 .
niyal .P. . . harma . ha dhery
. . in h
. Flowering
Plants of ttarakhand (A Checklist). Bishen Singh Mahendra Pal Singh
Dehradun, 404pp.
. ystematic ositions of the ta a re orted in this comm nication accordin to AP
nformal ro
aertn.
rder amily
system of classi cation
en s
ecies
Angiosperms: Eudicots
Ranunculales: Menispermaceae
Tiliacora
acuminata
Angiosperms: Eudicots: Rosids: Fabids
Fabales: Fabaceae (Leguminosae)
Dunbaria
glandulosa
Angiosperms: Eudicots: Rosids: Fabids
Fabales: Fabaceae (Leguminosae)
Flemingia
lineata
Angiosperms: Eudicots: Core Eudicots
Saxifragales: Saxifragaceae
Saxifraga
minutissima
Angiosperms: Eudicots: Asterids: Lamiids
Gentianales: Apocynaceae
Oxystelma
esculentum
Angiosperms: Eudicots: Asterids: Lamiids
Lamiales: Acanthaceae
Hygrophila
ringens
Angiosperms: Monocotyledons: Commelinids
Zingiberales: Zingiberaceae
Alpinia
nigra
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6101–6107
hreatened a a
o rnal of hreatened a a
Part of the activities of
the Institute of Ayurveda and
Integrative Medicine, Bengaluru
(formerly known as Foundation
ISSN
Online 0974–7907
for Revitalization of Local Health
Print 0974–7893
Traditions
(FRLHT))
who’s
P A
Repository of Medicinal Resources’
(RMR) houses over 30,000 voucher
specimens comprising around 4,200 species (3100
of them are medicinal), we carried out an extensive
botanical survey in Paderu, Narsipatnam and Chintapalli
forest ranges (deciduous to moist deciduous forests)
in Visakhapatnam District, Andhra Pradesh during
September 2011, which resulted in the documentation
of 142 taxa of flowering plants. During the process
of verification and authentication of the herbarium
specimens with the help of Revisions and Floras by
Hooker (1872–1897), Gamble (1915–1936), Saxena &
Brahman (1994), Pullaiah (1997), Pullaiah & Ali Moulali
(1997), Pullaiah & Chennaiah (1997) and Rao & Kumari
(2003, 2008) we found 18 taxa were of distributional
importance. Of these, two are new reports to southern
India, two new reports to the state of Andhra Pradesh as
well as to the Eastern Ghats, seven taxa form additions
to the Visakhapatnam District; seven are endemic.
The following exotic species namely Clerodendrum
aculeatum (L.) Schltr. (Verbenaceae), Stachytarpheta
cayennensis (Rich.) Vahl (Verbenaceae), Ipomoea
bona-nox L. (Convolvulaceae), Nicandra physalodes (L.)
Gaertn. (Solanaceae) and omordica charantia L. var.
.threatenedta a.or
ly
A
P
. a i mar
. alachandran . oor nnisa
e m P. Patchaimal Manoran an han a
. ohitasy d
1,2,3,4,
National Herbarium of Medicinal Plants and Repository of
Raw Drug, Institute of Ayurveda and Integrative Medicines (I-AIM),
Foundation for Revitalisation of Local Health Traditions (FRLHT), No.
74/2, Jarakabande Kaval, Post Attur, Via. Yelahanka,
Bengaluru, Karnataka 560106, India
5
Additional PCCF, 5th Floor, Aranya Bhavan, Saifabad, Hyderabad,
Telangana 500001, India
6
District Forest O cer, Guntur, Seemandhara 522004, India
1
k.ravikumar frlht.org (corresponding author), 2 nbala plant frlht.org,
3
noorunnisa.begum frlht.org, 4 patchaimal gmail.com,
5
mrbhanja gmail.com, 6 lohit.kadali gmail.com
muricata (Willd.) Chakrav. (Cucurbitaceae) have also
been collected from the study area in wild habitats.
These taxa are presented in alphabetical order with
brief botanical descriptions, distribution and important
field/taxonomical notes, if any. All the herbarium
specimens are deposited at the herbarium of FRLHT
(FRLH).
Adenia cardiophylla (Mast.) Engl. in Bot. Jahrb. Syst.
14: 376. 1891; N. Rama Rao et al. in J. Econ. Tax. Bot. 9:
241–245. 1987; Vasudeva Rao in J. Econ. Tax. Bot. 18:
243–244, 1994; Pull. & Chennaiah, Fl. Andhra Pradesh
http://dx.doi.org/10.11609/JoTT.o3144.6108-21
ditor B. Ravi Prasad Rao, Sri Krishnadevaraya University, Anantapur, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3144 | Received 30 March 2012 | Final received 07 July 2014 | Finally accepted 10 July 2014
itation Ravikumar, K., N. Balachandran, S.N. Begum, P. Patchaimal, M. Bhanja & K. Lohitasyudu (2014). Interesting plant records from Visakhapatnam District,
Andhra Pradesh, India. Journal of Threatened Taxa 6(8): 6108–6121; http://dx.doi.org/10.11609/JoTT.o3144.6108-21
o yri ht © Ravikumar et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
ndin This study is the outcome of botanical survey supported by Ministry of Environment and Forests, Government of India, New Delhi.
Sanction number: (13-06/2007-CS.I).
om etin nterest The authors declare no competing interests.
Ac no led ements The authors are thankful to the Director, FRLHT and Shri DK Ved, IFS (Retd.) Advisor, FRLHT, Bangalore for providing facilities, constant
support and encouragements; the Principal Chief Conservator of Forests, Forest Department, Hyderabad, Andhra Pradesh for granting permission and providing
logistic supports during the field survey; the Ministry of Environment and Forests, New Delhi for financial support under Centre of Excellence project; the Forest
Department o cials at Visakhapatnam, Paderu and Narasipatnam ranges for help during field visits.
lant records from isa ha atnam istrict
a i
mar et al.
© K. Ravikumar
nterestin
ma e . Adenia cardiophylla
1: 403. 1997. Modecca cardiophylla Mast. in Hook. f. Fl.
Brit. India 2: 602. 1879. (Images 1,2) (Passifloraceae).
Stout climber; stems terete, glabrous. Leaves remote,
broadly ovate, deeply cordate at base, acute to shortly
acuminate at apex, basally five nerved, secondary veins
parallel, ca. 14.5x16 cm; petioles with a sessile gland
on either side towards apex. Fruiting peduncles ca. 10
cm long, terminal part modified into tendril. Fruit a
3-valved capsule, tapering at both ends, ca. 5.7x3 cm,
green turning yellow and dark red when ripe, shiny,
ovoid; seeds 14–27, flat, pitted, sub-reniform, covered
by white aril, attached by 10–12 mm long funicles.
Specimen examined: 111334, 28.ix.2011, (in fruit),
elevation at 1200m, G. Madugula Village, Chodavaram
Taluk, Visakhapatnam District, Andhra Pradesh, India,
coll. K. Ravikumar & N. Balachandran.
Distribution: India (peninsular and northeastern
Regions, Andaman and Nicobar Islands), Bhutan,
Myanmar, China (Yunnan), Thailand, Cambodia, Laos,
Vietnam and Malaysia.
Notes: In India this species has so far been known from
northeastern states such as Sikkim, Assam Meghalaya
(Khasi Hills) and Andaman & Nicobar Islands. Rama Rao
et al. (1987) collected this species from Rampa Hills Madhiveedu, East Godavari District, Andhra Pradesh
and reported it as a new record to southern India. They
further stated that it is rare on the bushes near streams
in mixed forests; at an altitude between 1000–2000 m.
The present collection from Visakhapatnam extends
its distribution further towards north of its earlier
record from East Godavari District and also forms first
report to the district. Only three plants have been
noticed climbing on bushes along the road side. It is
associated with Sterculia villosa Roxb. Cassia stula L.
and Thysanolaena latifolia (Roxb. ex Hornem.) Honda.
ma e . erbari m of Adenia cardiophylla
Destruction of the nearby forest areas for cultivation in
future is a serious threat to the existence of this species.
Amorphophallus bulbifer (Roxb.) Blume in Rumphia
1:148.1837; Hook. f., Fl. Brit. India 6: 515.1893; C.E.C.
Fisch. in Fl. Pres. Madras 3: 1598 (1107). 1931; Pull.,
Fl. Andhra Pradesh 3: 1022. 1997; R.S. Rao, Fl. East
Godavari. 719. 1999. Arum bulbiferum Roxb., Fl. Ind.ed.
1832. 3: 510. 1832. (Image 3) (Araceae).
Erect herb. Tuber globose. Leaves up to 85cm in diam.
3-fid, decompound; odd pinnae up to 45cm, obovatelanceolate; leaflets oblanceolate-oblong, sessile, apex
long acuminate-caudate, lower leaflets smaller, upper
longer, 5.5x2.5 to 21x7.5 cm; with brownish, depressed
globose, bulbilliferous at the axial of every fork; petioles
up to 1.2m long, mottled with brown, white and green
spots and streaks.
Specimen examined: 111326, 28.ix.2011, (in
vegetative form), elevation at 1128m, Paderu Taluk,
Minumuluru, Visakhapatnam District, Andhra Pradesh,
India, coll. K. Ravikumar & N. Balachandran.
Distribution: India (northeastern India and the
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
mar et al.
ma e . erbari m of Ardisia depressa
ma e . erbari m of Amorphophallus bulbifer
Himalayan region) and Myanmar.
Notes: This plant has been represented by a single
collection at CAL for the State of Andhra Pradesh from
Devarakonda, the hills of East Godavari District. The
present collection could be the second collection
for Andhra Pradesh and forms as a new report to
Visakhapatnam District.
Ardisia depressa C. B. Clarke in Hook. f., Fl. Brit. India
3: 522.1882; Gamble, Fl. Madras 2: 755.1921; Haines,
Bot. Bihar & Orissa 509. 1922; Haines, Suppl. Bot. Bihar
& Orissa 84. 1950; Pull. & Moulali, Fl. Andhra Pradesh
2:547.1997; Subba Rao & Kumari, Fl. Visakhapatnam 1:
473. 2003. (Image 4) (Myrsinaceae).
Shrub, up to 3m tall; branchlets terete, glabrous.
Leaves elliptic-lanceolate, ca. 10x2.5 cm, glabrous with
punctuate glands on both sides; lateral nerves obscure.
Flowers in 1-3-flowered umbels, dull pink, ca. 1cm
across. Drupes globose, shiny, ca. 6mm across, ripening
black.
Specimen examined: 110884, 27.ix.2011, (in
flowers and fruits), elevation at 1130m, Paderu Taluk,
Minumuluru, Visakhapatnam District, Andhra Pradesh,
India, coll. K. Ravikumar & N. Balachandran.
Distribution: India (Andhra Pradesh, Assam, Bihar,
Kerala, Meghalaya, Orissa and Sikkim) and Myanmar.
Notes: The distribution of this species in India is
restricted to northeastern states (Sikkim, Meghalaya and
Assam) to Bihar, Orissa and Andhra Pradesh. In Andhra
Pradesh, it has been recorded only in Visakhapatnam
District. The distribution of this species is unique and the
occurrence is restricted to the highly fragile ecosystem
in the study area which needs immediate conservation
measures.
Aspidopterys indica (Willd.) W.Theob. Burmah ed.
3, 2: 599 1883; Hochr. in Bull. Inst. Bot. Buitenzorg 19.
45. 1904; Pullaiah & Chennaiah, Fl. Andhra Pradesh
1: 163.1997; R.C. Srivast. in Hajra et al., Fl. India 4: 8.
1997. Triopterys indica Willd. Sp. Pl., ed. 4 , 2(1): 744.
1799 Aspidopterys roxburghiana A. Juss. in Ann. Sci.
Nat. Bot. Ser. 2. 13: 267. 1840; Hook. f., Fl. Brit. India 1:
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
mar et al.
report to Visakhapatnam District.
Cassine paniculata (Wight & Arn.) Lobr.-Callen in
Adansonia (ser. 2) 15:220. 1975; Kostermans, A.J.G.H.
Gard. Bull. Singapore 39: 178. 1986; Nair & Henry. Fl.
Tami Nadu 1: 72. 1983; K. Ramamurthy in N.P. Singh et
al. (eds.) Fl. India 5: 83. 2000. Elaeodendron paniculatum
Wight & Arn., Prodr. Fl. Ind. 157. 1834; Gamble, Fl.
Madras 1:212. 1918; Matthew, Ill. Fl. Palni Hills t. 119.
1996. (Images 6,7) (Celastraceae).
Tree up to 10m tall. Leaves elliptic-lanceolate, ca.
10x4 cm, oblique at base, irregularly crenate along
margins. Inflorescense a dichasial corymbose cymes,
usually in upper leaf axils; peduncles up to 6cm long.
Flowers ca. 7mm across, greenish-yellow; petals
spathulate.
Specimen examined: 111386, 30.ix.2011, (in flower),
elevation at 550m, Rompulu Ghat Road (Pedavalasa–
Rinthada route), Visakhapatnam District, Andhra
Pradesh, India, coll. K. Ravikumar & N. Balachandran.
Distribution: India (Karnataka, Kerala and Tamil
Nadu), Sri Lanka and Malaysia.
420. 1874; Gamble, Fl. Madras 1: 129. 1915. (Image 5)
(Malphigiaceae)
Slender climber; young stem ferruginous hairy.
Leaves ovate-elliptic, ca. 12x8 cm, cordate to subcordate
at base, acute-acuminate at apex, glabrous above,
pubescent along nerves beneath. Inflorescense axillary,
corymbose panicles, ca. 12cm long, rusty pubescent.
Flowers white, 5–6 mm across; calyx and corolla
5-partite, pubescent outside; stamens 10; styles 3,
glabrous, unequal; stigma capitate.
Specimen examined: 111348, 29.ix.2011, (in
flower), elevation at 422m, Nimmalapalayam Village,
Narasipatnam Range, Visakhapatnam District, Andhra
Pradesh, India, coll. K. Ravikumar & N. Balachandran.
Distribution: India (Andhra Pradesh, Assam, Madhya
Pradesh, Meghalaya, Nagaland, Orissa, and Tamil Nadu)
and Myanmar.
Notes: Hooker (1872–1897) recorded the distribution
of this species in India and Birma (Myanmar), Saluen
River , whereas in Flora of India (1997) it is stated as
Endemic to India. The present collection forms a new
© K. Ravikumar
ma e . erbari m of Aspidopterys indica
ma e . Cassine paniculata
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
lant records from isa ha atnam istrict
a i
mar et al.
© K. Lohitasyudu
nterestin
ma e . Cleidion javanicum
ma e . erbari m of Cassine paniculata
Notes: In India, so far this species has been reported
only from the Western Ghats of Kerala, Karnataka and
Tamil Nadu. The present collection from Andhra Pradesh
not only forms a new report for the state but also for the
Eastern Ghats.
Cleidion javanicum Bl., Bijdr. 613. 1826; Hook.f., Fl.
Brit. India 5: 444. 1887; Airy-Shaw in Kew Bull. 36: 279,
f. 3D. 1981; N.P. Balakr. & Chakrab., Euphorbicaeae in
India 134. 2007. (Images 8,9) (Euphorbiaceae).
Tree, up to 10m tall; bark ashy brown, smooth.
Leaves alternate, elliptic-oblanceolate, up to 16x6.5 cm,
cuneate at base, abruptly acuminate at apex, distantly
crenate, glabrous above, domatia along the nerve axis
beneath; petioles ca. 10cm long. Female flowers solitary,
axillary; peduncles ca. 7cm long, thickened above; styles
2, each divided into 2 filiform arms. Capsules ca. 3cm
across, usually didymous.
Specimen examined:
110877, 27.ix.2011, (in
flowers), elevation at 1120m, Minumuluru, Paderu
Taluk, Visakhapatnam District, Andhra Pradesh, India,
coll. K. Ravikumar & N. Balachandran.
Distribution: India (Arunachal Pradesh, Assam, Bihar,
Karnataka, Kerala, Maharashtra, Manipur, Meghalaya,
Mizoram, Nagaland, Sikkim, Tamilnadu, Tripura, and A
& N Islands), Sri Lanka, Nepal, Bhutan, Myanmar, China,
ma e . erbari m of Cledion javanicum
Thailand, Malaysia and Australia.
Notes: In India, this species has so far been reported
in the northeastern states, throughout the Western
Ghats and the Andaman & Nicobar Islands (Balakrishnan
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
& Chakrabarthy 2007). The present collection forms
a new report for Andhra Pradesh state as well as the
Eastern Ghats.
mar et al.
a
© K. Ravikumar
Cynanchum corymbosum Wight, Contrib. Bot. India
56. 1834; Hook. f., Fl. Brit. India 4: 24. 1883; Kanjilal et
al. Fl. Assam 3: 286. 1939; Deb, Fl. Tripura 2: 31. 1983;
Jagtap & Singh in Fasc. Fl. India 24. 19. 1999. Cynoctonum
corymbosum (Wight) Decne. in DC., Prodr. 8. 528. 1844.
(Images 10,11,12). (Apocynaceae).
Slender, extensive climber; stems terete, pubescent
along two lines; latex white, thick. Stipules foliaceous,
reniform, ca. 1x1.3 cm. Leaves ovate-cordate, up to
20x12 cm, acuminate-caudate at apex, cordate at base
with incision up to 2cm deep looking like an inverted
U , glabrous above, glaucous beneath, with a distinct
sessile brown gland at the base of the lamina; petioles
ribbed, terete, ca. 4cm long. Flowers in corymbose
cymes, pubescent, white, fragrant; calyx-lobes 5, ovate,
short, ca. 1mm long; corolla-lobes 5, linear-lanceolate,
ca. 6x1 mm, greenish yellow; corona tubular, erect,
b
© K. Ravikumar
10
11
ma es
. Cynanchum corymbosum
ma e . erbari m of Cynanchum corymbosum
a o ers b fr its
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
white. Follicles generally in pairs, rarely single, ellipticlanceolate, ca. 10x5 cm, clothed with long dense fleshy
spines, echinations dull pink when young; seeds many,
ovate, brown with silky white coma.
Specimen examined: 111336, 28.ix.2011, (flowers
and fruits), elevation at 1045m, Sanjivini Vanam, Paderu
Taluk, Visakhapatnam District, Andhra Pradesh, India,
coll. K. Ravikumar & N. Balachandran.
Distribution: India (Arunachal Pradesh, Assam,
Manipur, Meghalaya, Nicobar, Sikkim, Tripura),
Cambodia, Laos, Malaysia, Myanmar and Vietnam.
Note: In India, this plant has so far been reported
only from the northeastern states (Arunachal Pradesh,
Assam, Manipur, Meghalaya, Sikkim and Tripura) and
Nicobar Islands. The present collection forms a new
report to southern India. Only one plant has been seen
growing along the side of a stream and found associated
with Hedychium coronarium J.Koenig, Mimosa pudica L.,
Dioscorea tomentosa J.Koenig ex Spreng, Cyclea peltata
(Lam.) Hook. f. & Thomson, Ardisia solanacea (Poir.)
ma e
. erbari m of Gardenia resinifera
mar et al.
Roxb., Neolitsea sp., Streblus taxoides (Roth) Kurz, etc.
Gardenia resinifera Roth, Nov. Pl. Sp. 150. 1821; Pull.
& Moulali, Fl. Andhra Pradesh 2: 475. 1997. (Image 13)
(Rubiaceae).
Large shrub, up to 4m high; bark greenish-grey;
branchlets terete. Leaves oblanceolate-elliptic, ca.
16x6.5 cm, shining, with prominent parallel nerves;
young buds with yellow resin. Fruits axillary, solitary,
ellipsoid ca. 2.5x1.5 cm, with persistent calyx-cup
consisting long, acuminate teeth.
Distribution: India (Western peninsula and East
Coast of Andhra Pradesh), Bangladesh and Myanmar.
Specimen examined: 110863, 26.ix.2011, (in
fruits), elevation at 30m, Kambala Konda Ecopark,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Notes: This plant is considered common in dry
deciduous forests in most districts of Andhra Pradesh
(Pullaiah & Moulali 1997), however there is no collection
from Visakhapatnam District (Rao & Kumarai 2008) and
therefore, it is an addition to that District.
Indigofera zollingeriana Miq., Fl. Ind. Bat. 1, 1: 310.
1885; Sanjappa, Legumes Ind. 198. 1992 & in Hajra et al.
Fasc. Fl. India 21. 159. 1995. Indigofera teysmanii Miq.,
Fl. Ind. Bat. 1: 108 3. 1855; C.J. Saldanha & G. Singh in
C.J. Saldanha, Fl. Karnataka 1: 474. 1984. Indigofera
benthamiana Hance in Ann. Sci. Nat. Bot. 4, 18: 219.
1862; Ravi in J. Bombay Nat. Hist. Soc. 73: 242. 1976.
(Image 14) (Fabaceae-Papilionoideae).
Tree up to 8m tall; branches horizontal and drooping.
Leaves odd pinnate, ca. 25x12 cm; leaflets lanceolate,
ca. 6.5x1.2 cm, obtuse at base, acute-acuminate at apex,
Inflorescence racemose, 10–21 cm long, perpendicular
to the branch. Flower pinkish red, ca. 1cm long, bud
ferruginous-brown outside. Pods ca. 4x0.3 cm, densely
packed in lower part of the peduncle and sparse in upper
part, straight, cylindrical with persistent thickened stylar
base, with brownish pubescent thick sutures; seeds
discoid, brown.
Specimen examined:
111328, 28.ix.2011, (in
flowers and fruits), elevation at 1100m, near Paderu,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Distribution: India (cultivated), China, Thailand, Laos,
Vietnam, Taiwan, Malaysia; cultivated in many countries
of Asia.
Note: Generally this plant is cultivated in coffee
and tea plantations and coconut groves. Occasionally
it is found as an escape in Andhra Pradesh, Gujarat,
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
lant records from isa ha atnam istrict
a i
mar et al.
© K. Ravikumar
nterestin
ma e
ma e
. Lasianthus truncatus
ma e
. erbari m of Lasianthus truncatus
. erbari m of Indigofera zollingeriana
Karnataka, Kerala, Tamil Nadu, Andaman & Nicobar
Islands, W. Bengal (Sanjappa 1995), but so far no records
from Andhra Pradesh.
Lasianthus truncatus Bedd., Ic. Pl. Ind. Or. t. 9. 1874;
Hook.f., Fl. Brit. India 3: 189.1880; Gamble, Fl.. Madras
2: 647. 1921; Deb & M. Gangop. in J. Econ. Taxon. Bot.
15(2): 265-308. 1991; H.O. Saxena & Brahmam, Fl. Orissa
2: 850.1995; Pull. & Moulali, Fl. Andhra Pradesh 2: 487.
1997. (Images 15,16) (Rubiaceae).
Shrub, up to 2m high; branchlets puberulous. Leaves
opposite, superimposed, narrowly lanceolate, ca. 15x3.5
cm, coriaceous, cuneate at base, acuminate-caudate at
apex; lateral nerves prominent on both sides, about
eight pairs. Flowers in sessile axillary clusters, ca. 6mm
across, white. Drupes subglobose to ovoid, ca. 9x6 mm,
glabrous, shiny, with a crown of calyx-cup, turbinate,
dark blue when ripe.
Specimen examined: 110883, 27.ix.2011, (in flowers
and fruits), elevation at 1130m, Minumuluru, Paderu
Taluk, Visakhapatnam District, Andhra Pradesh, India,
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
mar et al.
coll. K. Ravikumar & N. Balachandran.
Distribution: India (Eastern Ghats), Endemic.
Notes: Hooker (1872–1897) while providing the
distribution of this species quotes Beddome’s collection
from Mahendragiri Hills of Ganjam, Orissa at 1371m, and
Gamble (1921) refers the collection by A.W. Lushington
from the hills of Visakhapatnam at 1066m. Ours is the
fresh collection after almost a century. From this, it is
evident that this species is very rare.
The present collection was made in a small moist
forest patch surrounded by an abandoned estate. The
forest patch was about 200m long with a perennial
streamlet.
The biotic pressures such as timber
extraction, fuel wood collection and cattle grazing
were observed in this area which eventually poses a
serious threat to this patchy ecosystem along with a
few interesting floral elements. Only nine plants have
been counted in this fragile ecosystem and this species
is found growing along with Lasiococca comberi Haines,
Chassalia ophioxyloides (Wall.) Craib, Sarcococca saligna
Mull.Arg. and Amischotolype mollissima (Blume) Hassk.
Nervilia aragoana Comm. ex Gaudich. in Freycinet,
Voy. Uranie t. 15. 1827 & 422. 1829; Fischer, in Gamble
Fl. Madras 3: 1459. 1928; Pull. Fl. Andhra Pradesh 3. 950.
1997; R.S. Rao, Fl. East Godavari Dt. 665. 1999. (Image
17) (Orchidaceae).
Terrestrial, tuberous herb; tubers depressed
globose, ca. 1.6x1.5 cm. Leaves single, ca. 10x12 cm,
cordate to ovate, cordate at base, acuminate at apex,
wavy along margins, about 15-nerved from base, seven
pair of nerves on either side intersecting the main nerves
ending with in curved lobe along the margin; petioles up
to 21cm long.
Specimen examined:
111338, 28.ix.2011, (in
vegetative form), elevation at 1100m, Sanjivani Vanam,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Distribution: India (Himalayas, southern India),
Bangladesh, Bhutan, Indonesia, Japan, Laos, Malaysia,
Myanmar, Nepal, New Guinea, Philippines, Thailand,
Vietnam, Australia and Pacific Islands.
Notes: Though this species is reported to be
frequent under growth in moist forests of Rampa Hills,
in the adjacent East Godavari District (Rao et al. 1999),
in the present area only a few individuals have been
noticed, and this species also forms a new report for
Visakhapatnam District. This species has been assessed
as a Red Listed Medicinal plant by Ravikumar & Ved
(2000).
ma e
. erbari m of Nervilia aragoana
Ormocarpum cochinchinense (Lour.) Merr. in Philipp.
J. Sci. 5: 76.1910; Pull. & Chennaiah, Fl. Andhra Pradesh
1: 303. 1997. Diphaca cochinchinensis Lour. Fl. Cochinch.
454. 1790. Ormocarpum sennoides (Willd.) DC., Prodr.
2: 315.1825; Wight, Icon. Pl. Ind. Or. t. 297. 1840; Hook.
f. Fl. Brit. India 2: 152. 1876; Gamble, Fl. Madras 1: 332.
1918. (Fabaceae-Papilionoideae).
Shrubs, up to 2m high; branchlets glabrescent.
Leaves odd pinnate; leaflets about six pairs, alternate to
sub-opposite, obovate, ca. 1.5x1 cm, cuneate at base,
obtuse to emarginate at apex, chartaceous; petioles
minutely prickled.
Distribution: India (Peninsular) and Sri Lanka.
Specimen examined: 116947, 26.ix.2011, (in
vegetative form), at 30m, Kambala Konda Ecopark,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Notes: Rao & Kumari (2003) have not reported this
species in their Flora of Visakhapatnam and hence, it is
an addition to that District.
Pholidota pallida Lindl., Edw. Bot. Reg. 21: subt. 1777.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
mar et al.
1836; Hook., Exot. Fl. Sub t. 138. 1825; Hook. f., Fl. Brit.
India 5: 845.1890; Haines, Bot. Bihar Orissa 1167. 1924;
Fischer in Gamble, Fl. Madras 3:1431 (1000). 1928; Pull.,
Fl. Andhra Pradesh 3: 955.1997. (Orchidaceae).
Epiphytic, pendulous herb; pseudobulbs clustered,
ovate-cylindrical, 3–5 cm long. Leaves single, ellipticlanceolate, ca. 28x4 cm, acute at apex, basally 3-nerved,
narrowing in to a stout petiole. Inflorescence arise at top
of the pseudobulb, pendulous, ca. 45cm long. Flowers
ca. 3mm long, enclosed in persistent floral bracts.
Specimen examined: 116948, 28.ix.2011, (in
flowers and fruits), elevation at 1128m, Minumuluru,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Distribution: India (Northeastern India, Sikkim and
southern India) Nepal, Myanmar, China (Yunnan),
Thailand, Laos and Vietnam.
Notes: R.S. Rao collected this species from Gudem
Hills in Visakhapatnam District during 1964 (Pullaiah
1997) but Rao & Kumari (2003) have not included this
species in their Flora of Visakhapatnam. During the
present study it is seen on the tree trunks of Vitex quinta
(Lour.) F.N. Williams at Minumuluru, Paderu Taluk.
Pollia secundiflora (Blume) Bakh. f. in Baker, Beknopte
Fl. Java. Afl. a. Fam. 211, 10 (in clavi). 1949. var.
indica (Wight) Sanjappa in Fl. Ind. Enum. Monocot. 30.
1989; Pullaiah, Fl. Andhra Pradesh 3: 1012. 1997. Pollia
sorzogonensis (E. Mey) Endl., Gen. 1029.1840; Hook. f.,
Fl. Brit. India 6: 367. 1892; var. indica (Wight) Hook. f.;
Fischer in Gamble, Fl. Madras 3: 1536. 1931. A. indica
Wight, Ic, t. 2068. 1853. (Image 18) (Commelinaceae).
Perennial decumbent herb with ascending shoots.
Leaves lanceolate or elliptic-oblong, closely spaced
below the inflorescence, up to 24x7.5 cm, cuneate at
base, acuminate at apex, scabrous above, puberulous
below; sheaths tubular, viscid. Inflorescence terminal,
thyrsoid panicles, about 25cm long, Flowers ca. 8mm
across; perianth glabrous, clawed, white; bracts
persistent. Capsules ellipsoid, ca. 7x5 mm, shining blue;
seeds many, finely pitted.
Specimen examined: 110885, 27.ix.2011, (in
flowers and fruits), elevation at 560m, Minumuluru,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Distribution: India (peninsular India), Bhutan,
Myanmar, China, Malacca, Sri Lanka and New Guinea.
Notes: In India this species has been recorded
from the Deccan Peninsula (Hooker 1872). Gamble
(1931) while providing the distribution of this species
in southern India quotes the collections of Hohenacker
ma e
. erbari m of Pollia secundiflora
(Coorg), Meebold (S. Canara), Gamble (Wynaad),
Beddome (Anamalai & Tirunelveli) and Narayanaswami
(Ethakonda hills in Godavari). Pullaiah (1997) included
this taxon based on these earlier collections and Rolla
Rao’s collection from Gudem Hills in Visakhapatnam
District during 1964 (Pullaiah 1997.), but Rao & Kumari
(2003) have not included this species in their Flora of
Visakhapatnam District. In the present study about 25
individuals have been counted in an area of 10m2 and
seen growing along a shady and marshy place near a
streamlet. This area is often subjected to heavy biotic
interference.
Raphistemma pulchellum (Roxb.) Wall., Pl. As. Rar.
2:50, Pl. 163. 1831; Hook. f., Fl. Brit. India 4: 19.1883;
Brandis, Indian Trees 469. 1906; Kanjilal et al. Fl. Assam
3: 284. 1939; Jagtap & Singh in Fasc. Fl India 24: 45.
1999. Asclepias pulchella Roxb., Fl. Ind. ed. 1832 2: 54.
1832. (Images 19, 20) (Apocynaceae).
Twining, glabrous shrub; stems branched. Leaves
ca. 10x6 cm, broadly ovate, apiculate at apex, cordate
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
lant records from isa ha atnam istrict
a i
mar et al.
© K. Ravikumar
nterestin
ma e
. Raphistemma pulchellum
ma e
Rhamnus nepalensis (Wall.) M.A. Lawson in Hook.f.,
Fl. Brit. India.1: 640. 1875; H.O. Saxena & Brahmam,
Fl. Orissa 1: 312. 1994; Pull. & Chennaiah, Fl. Andhra
Pradesh 1: 204. 1997. Ceanothus nepalensis Wall. in
© K. Lohitasyudu
with incision 1–2 cm deep at base, basal lobes rounded,
glabrous above, sparingly pubescent along the veins
beneath; lateral veins 5–7 pairs, lower two arising from
the base of mid-vein, arched; petioles terete, 6–7.5 cm
long. Follicle single, ca. 10 x 2 cm, fusiform, turgid, with
a curved beak; seed many, silky white.
Specimen examined: 111312, 27.ix.2011, (in
fruits), elevation at 850m, Minumuluru, Paderu Taluk,
Visakhapatnam District, Andhra Pradesh, India, coll. K.
Ravikumar & N. Balachandran.
Distribution: India (Assam, Bihar, Orissa, Sikkim and
West Bengal), Java, Nepal.
Notes: In India, this species has so far been recorded
from the states of Assam, Sikkim, Bihar, West Bengal
and Orissa. The present collection forms a new report
for southern India. Only one plant has been seen
growing along Antidesma ghasembilla Gaertn., Ardisia
solanacea (Poir.) Roxb., Atalantia monophylla DC.,
Cyclea peltata (Lam.) Hook.f. & Thomson, Gnetum ula
Brongn., Gouania leptostachya DC., Lantana camara L.,
Tetrastigma sp. and Zanthoxylum armatum DC.
. erbari m of Raphistemma pulchellum
ma e
. Rhamnus nepalensis
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
Roxb., Fl. Ind. 2: 375. 1824. (Image 21) (Rhamnaceae).
Scandent shrub; branches unarmed, straggling,
glabrous on age. Leaves elliptic-oblong, ca. 14x5 cm,
obtuse at base, shortly acuminate at apex, distantly
crenate-serrate along margins, sub-coriaceous, glabrous;
lateral nerves about seven pairs; petioles about 2cm
long. Inflorescence an axillary clustered raceme, ca. 15
cm long; flowers cream, ca. 5mm across. Drupes ovoid,
ca. 6mm long, glabrous.
Specimen examined: 116949, 27.ix.2011, (in flowers
and fruits), elevation at 1130m, Minumuluru, Paderu
Taluk, Visakhapatnam District, Andhra Pradesh, India,
coll. K. Ravikumar & N. Balachandran.
Distribution: India (Andhra Pradesh, Assam, Bihar,
Orissa and West Bengal), Nepal, Myanmar, Malaysia and
China.
Notes: In India, this species has been reported from
the moist forests up to 1350m altitude in the Central
and Eastern Himalayan states and Bihar, Orissa, West
Bengal and Andhra Pradesh. Gamble (1925) reported
it from Mahendragiri Hill in Ganjam, Orissa at 1,500m
and Madgol Hills of Visakhapatnam at 1000m. Pullaiah
& Chennaiah (1997) stated that not even a single
specimen is found in CAL, MH and DD from Andhra
Pradesh . This indicates the rarity of this species and
therefore the present collection forms an important
contribution to Visakhapatnam District.
Only two plants have been noticed in this area and
are found associated with species like Gnetum ula
Brongn., Gouania leptostachya DC., Cardiospermum
halicacabum L. and Zanthoxylum armatum DC. This
plant looks very similar to Gouania leptostachya DC.
by its habit, branching pattern, phyllotaxy, shape and
size of the leaves, inflorescence pattern but can be
differentiated only by its un-winged drupes.
Senna sophera (L.) Roxb., Fl. Ind. 2: 347. 1832
(as sophora ); Larsen & Hou in Hou et al. Fl. Males.
12(2): 686. 1996; Singh, Monogr.Ind. Subtr. Cassiinae
(Caesalpiniaceae), 199. 2001. Cassia sophera L., Sp. Pl.
379. 1753; Pull. & Chennaiah, Fl. Andhra Pradesh 1: 204.
1997. (Image 22) (Fabaceae-Caesalpinioideae).
Shrub, up to 2m high. Leaves up to 20cm long;
leaflets 5–9 pairs, ovate or elliptic-lanceolate, sparsely
pubescent; petiole glands single. Inflorescence axillary
corymb; flowers ca. 2cm across; fertile stamens 7.
Specimen examined: 111384, 30.ix.2011, (in flower),
elevation at 550m, Rompulu Ghat Road (Pedavalasa–
Rinthada route), Visakhapatnam District, Andhra
Pradesh, India, coll. K. Ravikumar & N. Balachandran.
Distribution: India (Arunachal Pradesh, Goa, Gujarat,
a i
ma e
mar et al.
. erbari m of Senna sophera
Jammu Kashmir, Kerala, Rajasthan, Sikkim, Tamil Nadu,
Tripura, and Andaman & Nicobar Islands), Pakistan,
Nepal, Bhutan, Sri Lanka, China, Malysia, Indonesia,
Philippines, Africa and America; cultivated in many
other countries.
Notes: Pullaiah & Chennaiah (1997) state that this
species is common in all plain districts, by road sides
and on waste lands , but Flora of Visakhapatnam
District has no record of its presence, hence this is a new
addition to the district.
Sloanea sterculiacea (Benth.) Rehder & Wilson in
Sarg., Pl. Wilson. 2:362. 1915, p.p.; Murti in Sharma &
Sanjappa, Fl India 3: 566. 1993; Pull. & Chennaiah, Fl.
Andhra Pradesh 1: 160. 1997; Subba Rao & Kumari, Fl.
Visakhapatnam 1: 120.2003. Echinocarpus sterculiaceus
Benth. in J. Linn. Soc. 5 (Suppl. 2): 72. 1861; Masters
in Hook.f., Fl. Brit. India 1: 400. 1874. (Images 23–26)
(Elaeocarpaceae).
Deciduous trees, up to 20m tall; trunk buttressed;
bark smooth, brown, thick, with lichen patches
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
a i
mar et al.
© K. Ravikumar
23
24
© K. Lohitasyudu
ma e
© K. Ravikumar
25
ma es
. Sloanea sterculiacea
. erbari m of Sloanea sterculiacea
throughout branchlets puberulous. Leaves elliptic to
oblong-oblanceolate, up to 25x8.5 cm, cuneate-rounded
at base, acuminate at apex, glabrous, pubescent along
main nerve above; Inflorescence a fascicles arising
on leafless parts, tomentose; flowers ca. 3cm across,
greenish yellow; petals fringed, glandular disc at throat,
orange; stamens many, densely hairy, mucronate.
Capsules ovoid to globose, ca. 6cm across, covered
with numerous stiff spines. Seeds 4, black, shiny, with
orange-yellow aril.
Specimen examined: 111321, 28.ix.2011, (in flower
and fruit), at 1128m, Minumuluru, Visakhapatnam
District, Andhra Pradesh, India, coll. K. Ravikumar & N.
Balachandran.
Distribution: India (Uttar Pradesh, West Bengal,
Sikkim, Assam, Meghalaya and Manipur), Nepal, Bhutan,
Bangladesh, Myanmar and China (Yunnan).
Notes: Murti (1993) while providing the distribution
of this species in India mentioned the states namely
Uttar Pradesh (Kumaon, presently in Uttrakhand), Sikkim
and Assam. However, it has been later reported from
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
nterestin
lant records from isa ha atnam istrict
Visakhapatnam District by Pullaiah & Chennaiah (1997)
and Rao & Kumari (2003). The disjunctive occurrence
of this relict species in the Eastern Ghats is quite
interesting. Along the side of a stream one matured
tree about 1.5m gbh and a young one (about 7m tall
and about 30cm gbh) have been noticed. This location
faces a serious threat from biotic pressures, therefore
an immediate conservation measure is warranted.
eferences
eb . . M. an o adhaya
. Taxonomic study of the genus
Lasianthus Jack (Rubiaceae) in India. Journal of Economic and
Taxonomic Botany 15(2): 265–308.
amble . .
. . . ischer
. Flora of the Presidency
of Madras, Parts 1–11 (parts 1–7 by Gamble and 8–11 by Fischer).
Adlard & Sons Ltd., London, 1–2017pp.
a ra P. . P. . . ao eds.
. Flora of Great Nicobar Island.
Botanical Survey of India, Calcutta, 438pp.
oo er . . eds.
. Flora of British India ols.
. Reeve
& Co., London, (vol.i - 740pp; vol.ii - 792pp; vol.iii - 712pp; vol.iv 780pp; vol.v - 910pp; vol.vi - 792pp; vol.vii - 842pp).
a i
mar et al.
P llaiah .
. hennaiah
. Flora of Andhra Pradesh: Volume
1. Scientific Publishers, Jodhpur, 463pp.
P llaiah .
. Flora of Andhra Pradesh: Volume 3. Scientific
Publishers, Jodhpur, 922–1349pp.
P llaiah .
.A. Mo lali
. Flora of Andhra Pradesh: Volume 2.
Scientific Publishers, Jodhpur, 464–921pp.
amam rthy .
. Celastraceae, pp. 75–137. In: Singh, N.P., J.N.
Vohra, P.K. Hajra & D.K. Singh (eds.). Flora of India - Vol. 5. Botanical
Survey of India, Calcutta.
ao . . .
. . mari
. Flora of Visakhapatnam District,
Andhra Pradesh - Volume 1. Botanical Survey of India, India.
Ministry of Environment and Forests, Kolkata, 612pp.
ao . . .
. . mari
. Flora of Visakhapatnam District,
Andhra Pradesh - Volume 2. Botanical Survey of India, India.
Ministry of Environment and Forests, Kolkata, 536pp.
ao . . . dha ar P. en anna
. Flora of East Godavari
District. Indian National Trust for Art and Cultural Heritage (INTACH,
New Delhi), Andhra Pradesh State Chapter, Hyderabad, 1-947pp.
a i mar .
. . ed
. Illustrated Field Guide to 100 Redlisted edicinal Plants of Conservation Concern in Southern India.
Foundation for Revitalisation of Local Health Traditions, Bangalore,
467pp.
a ena . .
M. rahmam
. Flora of Orissa - Volume
(
). Orissa Forest Development Corporation Ltd.,
Bhubaneswar, 1–2918pp.
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6108–6121
o rnal of hreatened a a
In India much of the
lichenological investigations are
restricted to either Himalayan or
Western Ghats region. Systematic
ISSN
Online 0974–7907
studies in the recent times indicated
Print 0974–7893
that the Deccan Plateau and the
P A
Eastern Ghats also have a rich
diversity of lichens; Nayaka et al.
(2013) estimated the occurrence of about 180 species in
these areas. Keeping this in view a thorough exploration
of lichens is being undertaken in Andhra Pradesh. Reddy
et al. (2011) compiled the earlier studies on lichens of
Andhra Pradesh and enumerated 43 species for the state.
In continuation of the same study Nayaka et al. (2013)
reported a total of 26 new records for Andhra Pradesh
including a new record for India (Peltula farinosa B del).
It is now clear that Andhra Pradesh records a total of 69
lichen species.
The current study is restricted to lichen exploration
in the Rayalaseema region which includes four districts;
Anantapur, Chittoor, Kadapa and Kurnool. The area is
interesting in terms of its unique biodiversity as the
major tract of the Eastern Ghats passes through the
region with 21.38% forest cover. A large number of
lichen specimens were collected from these areas which
resulted in several interesting taxa. Out of these a total
of 10 species are reported here as new records for
Andhra Pradesh.
M
M
The morphological features
of lichen thallus and ascomata were observed under
Leica S8AP0 stereozoom microscope. Spot test for
colour reaction were carried out by 10% aqueous
solution of potassium hydroxide (K), Steiner’s stable
para-phenylenediamine solution (PD) and Calcium
hypochlorite solution (C). For anatomical investigation
.threatenedta a.or
ly
A
P
atish Mohabe A. Madh s dhana eddy . An ali
e i an ee a aya a
P. handramati han ar
Department of Botany, Yogi Vemana University, Vemanapuram,
Kadapa, Andhra Pradesh 516003, India
4
Lichenology Laboratory, CSIR-National Botanical Research Institute,
Rana Pratap Marg, Lucknow, Uttar Pradesh 226001, India
5
Department of Biotechnology, Yogi Vemana University,
Vemanapuram, Kadapa, Andhra Pradesh, 516003, India
1
satish.nbri09 gmail.com, 2 grassced yahoo.com, 3 anjalidevi634
gmail.com, 4 nayaka.sanjeeva gmail.com (corresponding author),
5
pchandra20 gmail.com
1,2,3
of fruiting bodies Leica DM500 compound microscope
was used. All the measurements of anatomical
structures were taken in water. The lichen substances
were identified with Thin Layer Chromatography in
solvent system A’ following White & James (1985).
The other literature followed for identification include
Awasthi (1991), Joshi (2008), Mayrhofer et al. (1996),
Nayaka (2005), Upreti (1994). Further, Lumbsch &
Huhndorf (2010) was followed for nomenclature and
classification, while Singh & Sinha (2010) was consulted
for distribution of the taxa. Identified specimens were
labelled, documented, digitalized and preserved at the
herbarium in the Department of Botany at Yogi Vemana
University (YVUH), Kadapa and voucher specimens were
deposited at the herbarium of CSIR-National Botanical
Research Institute, Lucknow (LWG).
It can be noted that in the two earlier studies
(Reddy et al. 2011; Nayaka et al. 2013) mostly foliose
lichens are included. In the present study, species being
reported are mostly crustose and squamulose forms.
http://dx.doi.org/10.11609/JoTT.o3726.6122-6
ditor G.P. Sinha, Botanical Survey of India, Allahabad, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3726 | Received 27 July 2013 | Final received 20 June 2014 | Finally accepted 08 July 2014
itation Mohabe, S., A.M. Reddy, B.A. Devi, S. Nayaka & P.C. Shankar (2014). Further new additions to the lichen mycota of Andhra Pradesh, India. Journal of
Threatened Taxa 6(8): 6122–6126; http://dx.doi.org/10.11609/JoTT.o3726.6122-6
o yri ht © Mohabe et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin Council of Scientific and Industrial Research, New Delhi.
om etin nterest The authors declare no competing interests.
Ac no led ements The Authors are grateful to Director, CSIR-National Botanical Research Institute, Lucknow for providing laboratory facilities and Dr. D.K.
Upreti, for his kind help and encouragement during the identification of lichens. Council of Scientific and Industrial Research, New Delhi is thanked for financial
support under sponsored scheme. Authors also thank Forest O cials of Andhra Pradesh for their cooperation during the study.
Additions to lichen mycota of Andhra Pradesh
Except for L. psuedistera and D. tenuis all the other
species were found growing on the bark of various trees.
The species L. psuedistera and D. tenuis not only have
saxicolous habitat, but also have squamulose to lobate
thallus.
. Biatorella conspersa
e ain. (Biatorellaceae)
Image 1A.
Specimen examined: 2348 (YVUH), 25.vi.2012,
elevation 328m, on bark, 8km from Diguvamitta on
the way to GBM, Vankamanu Gundla, Kurnool District,
Andhra Pradesh, coll. A. Madhusudhana Reddy.
This crustose, corticolous species is characterized by
greenish-yellow to yellow or yellowish-orange, granular
sorediate thallus; sessile 0.3–0.6 mm diameter, biatorine
apothecia, yellow pruinose disc; multispored asci with
hyaline rounded to globose, 1–3 0.5–2.5 m spores.
The species is known from Australia, Nepal and in
India it was earlier reported from Manipur.
. Caloplaca bassiae
illd. e Ach.
ahlbr.
(Teloschistaceae) Image 1B.
Specimen examined: 2009 (YVUH), 12.vi.2012, on
bark, Horsley hills, Chittoor District, Andhra Pradesh,
coll. A. Madhusudhana Reddy, Anjali Devi B. & Sanjeeva
Nayaka.
This crustose, corticolous, greenish-yellow to
yellowish-orange species is characterized by numerous
yellowish-orange, simple to coralloid branched
isidia; rare, scattered, sessile, 0.3–0.8 mm diameter,
biatorine apothecia with orange to brownish-orange
disc, sometimes isidiate, paler margin; K purple
epihymenium; 8-spored asci with 10–15 4–8 m spores
and with parietin as lichen substance.
The species is known from tropical America and
Nepal. In India, it was earlier reported from Andaman
& Nicobar Islands, Arunachal Pradesh, Assam, Bihar,
Himachal Pradesh, Jammu & Kashmir, Jharkhand,
Madhya Pradesh, Odisha, Rajasthan, Sikkim, Tamil Nadu
and Uttar Pradesh.
. Caloplaca poliotera yl. tein (Teloschistaceae)
Image 1C.
Specimen examined: 1850/A (YVUH), 13.vi.2012,
elevation 746.5m, on bark, Japali Anjneya Swami
Temple, Chittoor District, Andhra Pradesh, coll. A.
Madhusudhana Reddy & Sanjeeva Nayaka.
This crustose, saxicolous species is characterized
by greenish-grey to grey, rimose areolate thallus with
black prothallus; numerous, rounded, sessile apothecia
of size 0.2–0.5 mm in diameter, mostly present at the
centre of the thallus; yellowish to reddish-brown disc,
Mohabe et al.
biatorine to lecidine, brownish to black margin; K
purple epihymenium, absence of algal cells in exciple;
8-spored asci; hyaline, polaribilocular, elongate to
ellipsoidal, 12.0–14.0 8.0–9.5 m ascospores and with
anthraquinons as lichen substance.
The species is known from the tropical regions of the
world and in India it was earlier reported from Madhya
Pradesh, Tamil Nadu and West Bengal.
. Dimelaena tenuis M ll. Ar . . Mayrhofer
le (Physciaceae) Image 1D.
Specimen examined: 2178 (YVUH), 15.vii.12, on
rock, backside of Javakaladinnae, Gorantla, Anantapur
District, Andhra Pradesh, coll. A. Madhusudhana Reddy.
This e gurate, squamulose species, found growing
tightly on rock and characterized by yellowish-green to
greenish-brown central part and greenish to yellowish
or brownish marginal area, rhizines lacking; innate to
sessile, rounded to irregular, 0.3–0.7 mm in diameter,
biatorine to lecanorine apothecia, dark brown to
brown black disc; 8-spored asci; brown, 1 septate, 9.0–
11.0 4.0–7.0 m ascospores and with gyrophoric acid as
lichen substance.
The species is known from North America and in
India it was earlier reported from Madhya Pradesh.
i
. Lecanora chlarotera
yl. (Lecanoraceae) Image
1E.
Specimens examined: 1806/B (YVUH), 13.vi.2012, on
bark, on the backside of the arch, Shilathoranam, Chittoor
District, Andhra Pradesh, coll. A. Madhusudhana Reddy
& Sanjeeva Nayaka; 1822 (YVUH), 13.vi.2012, elevation
746.5m, on bark, Japali Anjneya Swami Temple, coll. A.
Madhusudhana Reddy & Sanjeeva Nayaka.
This crustose, corticolous species characterized
by greenish-grey to grey, verruculose to verrucose
thallus; numerous, 0.2–0.9 mm in diameter, lecanorine
apothecia, pale orange to orange brown or reddish brown
disc; large crystals and algal cells in exciple, yellowish to
brownish epihymenium dissolving in K; 8-spored asci;
simple to ellipsoidal, 11.0–15.0 8.0–10.0 m ascospores
and with atranorin, zeorin as lichen substance.
The species is widely distributed in Asia, Europe and
America. In India it was earlier reported from Jammu
& Kashmir, Karnataka, Maharashtra, Manipur, Nagaland,
Rajasthan, Tamil Nadu, Uttarakhand and West Bengal.
. Lecanora helva ti enb. (Lecanoraceae) Image 1F.
Speciemen examined: 1896 (YVUH), 12.vi.2012, on
bark, Horsley hills, Chittoor District, Andhra Pradesh,
coll. A. Madhusudhana Reddy, Sanjeeva Nayaka & B.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6122–6126
Additions to lichen mycota of Andhra Pradesh
Mohabe et al.
A
D
F
ma e . A Biatorella conspersa
Caloplaca bassiae
Caloplaca poliotera
A
- Lecanora chlarotera
Lecanora helva
. Scale bars: A, B, C, D, F 0.5mm, E 1mm.
Dimelaena tenuis
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6122–6126
Additions to lichen mycota of Andhra Pradesh
Anjali Devi.
This crustose, corticolous species characterized by
greenish-grey to grey, smooth to verruculose thallus;
crowded, round, 0.2–1.0 mm in diameter, lecanorine
apothecia; pale yellow to brownish-yellow or orange
disc; large crystals and algal cells in exciple; yellowish to
brownish epihymenium dissolving in K; 8-spored asci;
simple, colourless, 8.0–11.0 5.0–7.0 m ascospores and
atranorin as lichen substance.
The species is distributed in Africa, Australasia, Pacific
regions, South America and Thailand. In India it was
earlier reported from Assam, Goa, Himachal Pradesh,
Kerala, Madhya Pradesh, Maharashtra and Tamil Nadu.
. Lecanora interjecta M ll. Ar . (Lecanoraceae)
Image 2G.
Specimens examined: 1817, 1818, 1824, 1839
(YVUH), 13.vi.2012, elevation 746.5m, on bark, Japali
Anjaneya Swami Temple, Chittoor District, Andhra
Pradesh, coll. A. Madhusudhana Reddy & Sanjeeva
Nayaka.
This crustose, corticolous species characterized
by greenish-grey, verruculose to verrucose thallus;
numerous, sessile, lecanorine, 0.2–1.0 mm in diameter,
apothecia, pale brown to orange brown disc; large
crystals and algal cells in exciple; yellowish to pale brown
epihymenium dissolving in K; 8-spored asci; hyaline,
ellipsoidal, 9.0–13.0 4.0–7.0 m ascospores and with
atranorin, usnic acid as lichen substances.
The species is distributed in Africa, Australia, Atlantic
Islands, Chile, Europe and New Zealand. In India it was
earlier reported from Arunachal Pradesh and Himachal
Pradesh.
. Lecanora pseudistera yl. (Lecanoraceae) Image
2H.
Specimens examined: 2136, 2139, 2143, 2159
(YVUH), 15.vii.2012, on rock, Javakaladinnae,
Gorantla, Ananthapur District, Andhra Pradesh, coll. A.
Madhusudhana Reddy; 1485, 2175 (YVUH), 14.i.2012, on
rock, backside of Javakaladinnae, coll. A. Madhsudhana
Reddy.
This crustose, saxicolous species characterized
by whitish-grey to grey, areolate to subsquamulose
thallus; 0.5–1.0 mm in diameter, lecanorine apothecia;
yellowish-orange to reddish-brown disc; small crystals
and algal cells in exciple; yellowish to orange brown
epihymenium dissolving in K; 8-spored asci; hyaline,
ellipsoidal, 8.0–11.0 5.0–7.0 m ascospores and with
atranorin, 2-O-methylperlatolic acid as lichen substance.
The species is known from all continents except
Mohabe et al.
Antarctica and in India it was earlier reported from
Himachal Pradesh, Karnataka, Madhya Pradesh,
Rajasthan, Tamil Nadu and Uttarakhand.
. Pertusaria melastomella yl. (Pertusariaceae)
Image 2I.
Specimen examined: 2009 (YVUH), 12.vi.2012, on
bark, Horsley hills, Chittoor District, Andhra Pradesh,
coll. A. Madhusudhana Reddy, Sanjeeva Nayaka & B.
Anjali Devi.
This crustose, corticolous species characterized
by whitish grey or greenish-grey, verrucose thallus;
perithecioid apothecia, 1–2 per verrucae; verrucae
not constricted at base, 0.3–0.4 mm high, 0.6–0.8 mm
wide; 6–8 spored asci; large, ellipsoidal, double walled,
smooth, 59.0–87.0 24.0–34.0 m ascospores.
The species is known from Sri Lanka and In India it
was earlier reported from Himachal Pradesh, Madhya
Pradesh and Tamil Nadu.
. Porina tetracerae Af . M ll. Ar . (Porinaceae)
Image 2J.
Specimen examined: 1826 (YVUH), 13.vi.2012,
elevation 746.5m, on bark, starting point on the left side
of rocky zone, Japali, Chittoor District, Andhra Pradesh,
coll. A. Madhusudhana Reddy & Sanjeeva Nayaka.
This crustose, corticolous species characterized by
greenish-brown to brown thallus; solitary, semiglobose
to globular perithecia; punctiform, pale brown
ostioles; slightly yellowish peridium, yellowish to
brown involucrellum; 6-spored asci; hyaline, fusiform,
transversely 1–7 septate, 34.0–44.0x6.0–8.0
m
ascospores.
The species is distributed in Brazil, Ecuador, French
Guiana, Mexico and Peru. In India it was earlier recorded
from Andaman & Nicobar Islands, Arunachal Pradesh,
Goa, Karnataka, Madhya Pradesh, Nagaland, Orissa,
Sikkim, Tamil Nadu and West Bengal.
A asthi . .
. A Key to the Microlichens of India, Nepal and
Sri Lanka. Bibliotheca Lichenologica, J. Cramer, Berlin, Stuttgart 40:
ii 337pp.
oshi
.
. Morphotaxonomic studies on lichen family
Teloschistaceae from India. PhD Thesis. University of Kumaun.
Nainital, India, 293pp.
mbsch . .
.M. hndorf
. Outline of Ascomycota - 2009.
Myconet 14: 1–64; http://dx.doi.org/10.3158/1557.1
Mayrhofer . M. Mat er A. i el
.A. li
. Genus
Dimelaena (Lichenized Ascomycetes, Physciaceae) in the Southern
Hemisphere. Mycotaxon 58: 293–311.
aya a .
. Revisionary studies on lichen genus Lecanora sensu
lato in India. PhD. Thesis. Dr. Ram Manohar Lohia Avadh University,
Faizabad, India, 241pp.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6122–6126
Additions to lichen mycota of Andhra Pradesh
Mohabe et al.
J
ma e .
Lecanora interjecta
Lecanora pseudistera
Scale bars: G 0.2mm, H, J 0.5mm, I 1mm.
aya a . M.A. eddy P. Ponm r an A. . e i . Ayya adasan
. .
reti
. Eastern Ghats, biodiversity reserves with
unexplored lichen wealth. Current Science 104(7): 821–825.
eddy M.A. . aya a P. . han ar . . eddy
. .P. ao
.
New distributional records and checklist of lichens for Andhra
Pradesh, India. Indian Forester 137: 1371–1376.
in h .P.
.P. inha
. Indian Lichens: Annotated Checklist.
Botanical Survey of India, Kolkata, 572pp.
Pertusaria melastomella
Porina tetracerae
.
reti . .
. Notes on corticolous and saxicolous species of
Porina from India with Porina subhibernica sp. nov. Bryologist 97(1):
73–79.
hite . . P. . ames
. A new guide to the microchemical
technique for the identification of lichen substances. British Lichen
Society Bulletin 57(suppl.): 1–41.
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6122–6126
o rnal of hreatened a a
A
.threatenedta a.or
A
C.K. Adarsh 1
A
. . Aneesh 2
P. . ameer 3
Centre for Wildlife Sciences, College of Forestry, Kerala Agricultural
University, Thrissur, Kerala 680656, India
2
Department of Forestry and Wood Technology, Sir Syed College,
Taliparamba, Kannur, Kerala 670142, India
1
adarshckcof09@gmail.com, 2 aneeshkscof@gmail.com,
3
nameer.po@kau.in (corresponding author)
1, 3
The order Odonata, which comprise of dragonflies and
damselflies are one of the fascinating groups of insects.
Because of their amphibious life history, relatively short
generation time, high trophic position, and diversity,
odonates are considered as an important component
of freshwater ecosystems as well as good indicators of
ecosystem health (Corbet 1993; Clark & Samways 1996).
There are numerous studies from the world, which have
documented that odonates respond to anthropogenic
activity and thus may serve as useful indicators of
habitat quality in terms of species occurrence and
distribution (Kadoya et al. 2004; Flenner & Sahlen 2008).
Globally 5,952 species of odonates have been reported,
of which 474 species in 142 genera and 18 families are
known from India (Subramanian 2014). Western Ghats
has 174 species of odonates (Subramanian et al. 2011),
while 154 species of odonates have been reported from
Kerala (Kiran & Raju 2011, 2013). Fraser in his three
volume treatises (1933, 1934 & 1936) on the odonates
ly
of the Indian subcontinent gave a
detailed account of the odonates
of Kerala also. The odonate fauna
of Kerala is well documented, some
ISSN
Online 0974–7907
of the prominent works include
Print 0974–7893
Peters (1981), Rao & Lahiri (1982),
P A
Prasad (1987), Mathavan & Miller
(1989), Radhakrishnan (1997),
Emiliyamma & Radhakrishnan (2000, 2002), Palot et
al. (2002), Subramanian & Sivaramakrishnan (2002),
Radhakrishnan & Emiliyamma (2003), Emiliyamma et al.
(2005), Subramanian (2005, 2007), Kiran & Raju (2011,
2013) which provided information regarding the status
and distribution of odonates in different parts of Kerala.
t dy area The Kerala Agricultural University (KAU)
main campus is located at Vellanikkara, Thrissur District,
Kerala (Fig. 1). The area lies between 10032’–10033’N
and 76016’–76017’E, with an average altitude of 50m.
KAU campus is located very close to the Peechi-Vazhani
Wildlife Sanctuary, Western Ghats, the aerial distance of
which is not more than 5km. The campus has a total
area of 391.44ha and the major habitats include garden
lands, botanical garden, plantations of rubber, coconut,
plantain, cocoa and orchards of mango, jack, sapota
and guava. The campus is also enriched with various
aquatic habitats like ponds, marshes, paddy fields, tanks
etc. (Appendix 1). KAU campus enjoys a moderate
climate. The last 10 year mean minimum temperature
is 23.30C and 10 year mean maximum of 31.80C. The
area receives south-west and north-east monsoons, the
greater portion of the rainfall, however is received from
the south-west monsoon between June and September.
The mean annual rainfall is 2,763mm. The mean number
of rainy days per year is 110 days (KAU weather station
http://dx.doi.org/10.11609/JoTT.o3491.6127-37 | oo an urn:lsid:zoobank.org:pub:07F4F866-20B6-406B-80AB-97D4767221D2
ditor K.A. Subramanian, Zoological Survey of India, Kolkata, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3491 | Received 17 January 2013 | Final received 08 June 2014 | Finally accepted 18 June 2014
itation Adarsh, C.K., K.S. Aneesh & P.O. Nameer (2014). A preliminary checklist of odonates in Kerala Agricultural University (KAU) campus, Thrissur District,
Kerala, southern India. Journal of Threatened Taxa 6(8): 6127–6137; http://dx.doi.org/10.11609/JoTT.o3491.6127-37
o yri ht © Adarsh et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin Kerala Agricultural University.
om etin nterest The authors declare no competing interests.
Ac no led ements We thank C.G. Kiran and David Raju, who confirmed the identification of odonates. We thank Sreehari R, for helping us in the preparation of
the map. The authors thank the three anonymous reviewers and the subject editor for their critical comments which greatly improved the manuscript. We also
thank the Dean, College of Forestry, Kerala Agricultural University for encouragement and support.
6127
Odonates in Kerala Agricultural University campus
i
Adarsh et al.
re . ocation ma of erala A ric lt ral ni ersity cam
s
ellani ara hriss r erala
2012).
Methods The odonates of the Kerala Agricultural
University campus were studied for one year from
February 2011 to March 2012. Surveys were conducted
throughout the campus to cover all the habitats.
Observations were done over three seasons viz.,
summer (March to May), monsoon (June to October)
and winter (November to February). Individual
specimens were photo-documented and these images
were cross-checked with standard references and field
guides on odonates such as Fraser (1933, 1934 & 1936),
Subramanian (2005, 2009) and Kiran & Raju (2013).
Systematic arrangement and the taxonomy followed in
the checklist is after Subramanian (2014) and common
names after Subramanian (2009). The odonate species
were categorized into the five relative abundance
categories such as very common (VC), those which were
sighted during 80-100 % of the field days, common (C)
(60–79 %), occasional (O), (40–59 %) and rare (R), (20–
39 %) and very rare (VR) for those that was sighted only
less than 19% of the field days.
es lts A total of 52 species of odonata including
36 species of Anisoptera (dragonflies) and 16 species of
Zygoptera (damselflies) were recorded from the Kerala
Agricultural University main campus, Thrissur, Kerala
(Table 1). The Libellulidae (29sp.) was the dominant
family among Anisoptera followed by Aeshnidae
(3sp.) and Gomphidae (3sp.). Among the Zygoptera
the dominant family was Coenagrionidae (8sp.)
followed by Calopterygidae (2sp.), Lestidae (2sp.) and
Platycnemididae (2sp.). The family wise distribution of
odonates is given in Fig. 2 and Fig. 3.
The relative abundance analysis shown that 21
species out of 52 were found to be occasional, 13 were
common, 10 very common, seven rare and one very
rare. Among Anisoptera, Green Marsh Hawk Orthetrum
sabina (Drury, 1770) and Common Picture Wing
Rhyothemis variegata (Linnaeus, 1763) were the most
common species, whereas among the Zygoptera, Yellow
Bush Dart Copera marginipes (Rambur, 1842) were the
common ones. The odonate diversity did not vary much
between the different seasons at KAU campus during
the study period (Fig. 4).
isc ssion Kiran & Raju (2011) reported 154 species
of odonates from Kerala. The present study on the
odonates of KAU main campus revealed the presence
of 52 species, which accounts 33.76% of total species of
odonates found in Kerala. The two dominant families
of odonates at KAU campus are Libellulidae, accounting
for 29 species and Coenagrionidae with eight species.
Earlier studies on the Kerala odonates from other
regions also have reported Libellulidae as the dominant
odonate family (Emiliyamma & Radhakrishnan 2000,
2002; Emiliyamma 2005; Emiliyamma et al. 2005). The
higher species diversity of odonates in KAU campus
may be attributed to the diverse ecosystems including
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
Odonates in Kerala Agricultural University campus
Adarsh et al.
able . hec list of odonates recorded from erala A ric lt ral ni ersity cam
ommon ame
A.
y o tera
read
s hriss r erala
amily cienti c name
ma e no.
stat s
Ab ndance
amsel ies
in
estidae
1
Emerald Spread wing
Lestes elatus Hagen in Selys, 1862
1
LC
R
2
Sapphire Eyed Spread wing
Lestes praemorsus Hagen in Selys, 1862
2
LC
O
3
Black-tipped Forest Glory
Vestalis apicalis Selys, 1873
3
LC
O
4
Clear Winged Forest Glory
Vestalis gracilis (Rambur, 1842)
4
LC
C
5
LC
R
6
DD
O
lories
alo tery idae
tream e el
5
hlorocy hidae
River Heliodor
orrent arts
6
Libellago lineata (Burmeister, 1839)
haeidae
Black Torrent Dart
amboo ail
Dysphaea ethela Fraser, 1924
Platycnemididae
7
Yellow Bush Dart
Copera marginipes (Rambur, 1842)
7
LC
VC
8
Black Bambootail
Prodasineura verticalis (Selys, 1860)
8
LC
R
Marsh art
oena rionidae
9
White Dartlet
Agriocnemis pieris Laidlaw, 1919
9
LC
C
10
Pigmy Dartlet
Agriocnemis pygmaea (Rambur, 1842)
10
LC
O
11
Orange-tailed Marsh Dart
Ceriagrion cerinorubellum (Brauer, 1865)
11
LC
O
12
Coromandel Marsh Dart
Ceriagrion coromandelianum (Fabricius, 1798)
12
LC
C
13
Orange Marsh Dart
Ceriagrion rubiae Laidlaw, 1916
13
14
Golden Dartlet
Ischnura aurora (Brauer, 1865)
14
LC
O
15
Blue Grass Dartlet
Pseudagrion microcephalum (Rambur, 1842)
15
LC
O
16
Saffron-faced Blue Dart
Pseudagrion rubriceps Selys, 1876
16
LC
O
B
Aniso tera
O
ra on ies
arners
Aeshnidae
17
Blue Darner
Anax immaculifrons Rambur, 1842
17
18
Parakeet Darner
Gynacantha bayadera Selys, 1891
18
LC
R
19
Brown Darner
Gynacantha dravida Lieftinck, 1960
19
DD
R
20
Spotted Lyretail
Heliogomphus promelas (Selys, 1873)
20
NT
R
21
Common Clubtail
Ictinogomphus rapax (Rambur, 1842)
21
LC
O
22
Common Hooktail
Paragomphus lineatus (Selys 1850)
22
LC
C
23
Common Torrent Hawk
23
LC
O
24
Trumpet Tail
Acisoma panorpoides Rambur, 1842
24
LC
O
25
Scarlet Marsh Hawk
Aethriamanta brevipennis (Rambur, 1842)
LC
C
26
Rufous-backed Marsh Hawk
Brachydiplax chalybea Brauer, 1868
25
LC
C
27
Little Blue Marsh Hawk
Brachydiplax sobrina (Rambur, 1842)
26
28
Ditch Jewel
Brachythemis contaminata (Fabricius, 1793)
27&28
LC
VC
29
Granite Ghost
Bradinopyga geminata (Rambur, 1842)
29
LC
VC
30
Black-tipped Ground Skimmer
Diplacodes nebulosa (Fabricius, 1793)
30
LC
C
31
Ground Skimmer
Diplacodes trivialis (Rambur, 1842)
31
LC
VC
32
Black Scrub Glider
Indothemis carnatica (Fabricius, 1798)
32
NT
C
l btails
orrent ha
VR
om hidae
s
immers
Macromiidae
Epophthalmia vittata Burmeister, 1839
ibell lidae
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
C
6129
Odonates in Kerala Agricultural University campus
Adarsh et al.
ommon ame
amily cienti c name
ma e no.
33
Amber-winged Marsh Glider
Hydrobasileus croceus (Brauer, 1867)
34
Asiatic Blood Tail
Lathrecista asiatica (Fabricius, 1798)
35
Fulvous Forest Skimmer
36
Pied Paddy Skimmer
37
38
stat s
Ab ndance
LC
O
33
LC
O
Neurothemis fulvia (Drury, 1773)
34&35
LC
VC
Neurothemis tullia (Drury, 1773)
36&37
LC
VC
Brown-backed Red Marsh Hawk
Orthetrum chrysis (Selys, 1891)
38
LC
O
Blue Marsh Hawk
Orthetrum glaucum (Brauer, 1865)
39
LC
O
39
Tricoloured Marsh Hawk
Orthetrum luzonicum (Brauer, 1868)
40
LC
O
40
Crimson-tailed Marsh Hawk
Orthetrum pruinosum (Burmeister, 1839)
41
LC
C
41
Green Marsh Hawk
Orthetrum sabina (Drury, 1770)
42
LC
VC
42
Wandering Glider
Pantala flavescens (Fabricius, 1798)
43
LC
VC
43
Yellow-tailed Ashy Skimmer
Potamarcha congener (Rambur, 1842)
44
LC
C
44
Rufous Marsh Glider
Rhodothemis rufa (Rambur, 1842)
45&46
LC
VC
45
Common Picture Wing
Rhyothemis variegata (Linnaeus, 1763)
47&48
LC
VC
46
Crimson Marsh Glider
Trithemis aurora (Burmeister, 1839)
49
LC
O
47
Pigmy Skimmer
Tetrathemis platyptera Selys, 1878
50
LC
48
Coral-tailed Cloud Wing
Tholymis tillarga (Fabricius, 1798)
51&52
LC
R
C
49
Black Marsh Torter
Tramea limbata (Desjardins, 1832)
53
LC
50
Long Legged Marsh Glider
Trithemis pallidinervis (Kirby, 1889)
54
LC
C
51
Greater Crimson Glider
Urothemis signata (Rambur, 1842)
55
LC
O
52
Brown Dusk Hawk
Zyxomma petiolatum Rambur, 1842
56
LC
O
O
LC - Least concern; NT - Near Threatened; DD - Data Deficient; VC - Very Common; C - Common; O - Occasional; R - Rare; VR - Very Rare.
8 30
8
7
6
5
4
3
2
1
0
8 20
15
10
6 Number of species mber of s ecies
5 4 3 2 2 2 2 1 1 1 hae
idae
Euphaeidae Eup
e
hida
rocy
p
ae
ycne
mid
id
ae
Chlorocyphidae Chlo
i re . amily ise distrib tion of dra on ies Aniso tera in A
am s
Platycnemididae Family Plat
Macromiidae
Les:dae Lesti
d
Gomphidae
amily
rion
Aeshnidae
nag
Libellulidae
Calopterygidae idae
Coenagrionidae idae
0 0
Calo
pter
yg
5
Coe
mber of s ecies
7 25
amily
mber of s ecies
i re . amily ise distrib tion of damsel ies y o tera in A
am s
35
30
25
20
15
10
5
0
Anisoptera
Zygoptera
Summer
Monsoon
easons
Winter
i re . ecies richness of odonates across the three seasons
s mmer monsoon and inter
agriculture fields and the water bodies present in the
campus. Sharma et al. (2007) reported that species
diversity of odonates would be higher in a diversified
ecosystem.
This study also reported two Near
Threatened species such as Heliogomphus promelas
(Selys, 1873) and Indothemis carnatica (Fabricius,
1798). The present study reiterates the significance of
KAU main campus in conserving the biodiversity of the
region. Earlier studies on the fauna of KAU main campus
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
Odonates in Kerala Agricultural University campus
have reported 135 species of birds (Nameer et al. 2000)
and 139 species of butterflies (Aneesh et al. 2013). This
is quite significant and thus emphasizing importance of
university campuses in biodiversity conservation.
eferences
Aneesh . . . . Adarsh P. . ameer
. Butterflies of Kerala
Agricultural University (KAU) campus, Thrissur, Kerala. India. Journal
of Threatened Taxa 5(9): 4422–4440; http://dx.doi.org/10.11609/
JoTT.o2870.4422-40
lar . . M. . am ays
. Dragonflies (Odonata) as indicators
of biotope quality in the Kruger National Park, South Africa. Journal
of Applied Ecology 33: 1001–1012.
orbet P.
. Are Odonata useful as bioindicators? Libellula 12(34): 91–102.
miliyamma . .
. On the Odonata (Insect) Fauna of Kottayam
District, Kerala, India. Zoo’s print Journal 20(12): 2108–2110; http://
dx.doi.org/10.11609/JoTT.ZPJ.1338.2108-10
miliyamma . .
. adha rishnan
. Odonata (Insecta)
of Parambikulam Wildlife Sanctuary, Kerala, India. Records of
Zoological Survey of India 98(1): 157–167.
miliyamma . .
. adha rishnan
. Additions to the
Odonata of (Insecta) of Thiruvananthapuram District, Kerala. Zoo’s
Print Journal 17(10): 914–917; http://dx.doi.org/10.11609/JoTT.
ZPJ.17.10.914-7
miliyamma . . . adha rishnan M. . Palot
. Pictorial
Handbook on Common Dragonflies and Damselflies of Kerala.
Zoological Survey of India, 67pp.
lenner .
. ahl n
. Dragonfly community re-organisation
in boreal forest lakes: rapid species turnover driven by climate
change? Insect Conservation and Diversity 1: 169–179.
raser . .
. The Fauna of British-India including Ceylon and
Burma, Odonata. Vol. I. Taylor and Francis Ltd., London, 436pp.
raser . .
. The Fauna of British-India including Ceylon and
Burma, Odonata. Vol. II. Taylor and Francis Ltd., London, 442 pp.
raser . .
. The Fauna of British-India including Ceylon and
Burma, Odonata. Vol. III. Taylor and Francis Ltd., London, 461pp.
adoya . . da
. ashitani
. Dragonfly species richness
on man-made ponds: effects on pond size and pond age on newly
established assemblages. Ecological Research 19(5): 461-467;
http://dx.doi.org/10.1111/j.1440-1703.2004.00659.x
iran . .
. . a
. Checklist of Odonata of Kerala with
their Malayalam names. Malabar Trogon 9(3): 31-35.
iran . .
. . a
. Dragonflies and damselflies of Kerala
(Keralathile Thumbikal). Tropical Institute of Ecological Sciences,
156p.
Adarsh et al.
Matha an .
P. . Miller
. A Collection of Dragonflies
(Odonata) made in the Periyar National Park, Kerala, South India,
in January 1988. International Odonatological Society, Bilthoven
(Rapid communications (supplements), no. 10), 10pp.
ameer P. . . . air . . Anoo
. . air . e shmi
P.
adha rishnan
. Birds of Kerala Agricultural University
Campus, Thrissur. Zoo’s Print Journal 15(4): 243–246; http://dx.doi.
org/10.11609/JoTT.ZPJ.15.4.243-6
Palot M. . . her at . . miliyamma
. adha rishnan
.
Dragonfly Menace at the National Fish Seed Farm, Malampuzha,
Kerala. Fishing Chimes 22(5): 56–60.
Peters .
. Trockenzeit-Libellen ausdem Indischen Tiefand.
Deutsch Entomologische Zeitschrift (N.F.) 28: 93–108.
Prasad M.
. A note on the odonata from south India. Fraseria
12: 50.
adha rishnan
.
. Ecology and conservation status of
Entomofauna of Malabar. Zoos’ Print 11: 2–5.
adha rishnan .
. . miliyamma
.Odonata (Insecta)
of Kerala: A systematic Database, pp. 1-27. In: Gupta, R.K. (ed.).
Advancement in Insect Biodiversity, Jai Narain Vyas University,
Jodhpur.
ao . A. . ahiri
. First records of Odonates (Arthropoda:
Insecta) from the Silent Valley and NewAmarambalam Reserved
Forests. Journal of the Bombay Natural History Society 79(3): 557–
562.
harma . . ndarara
. . aribas ara a
.Species diversity
of Odonata in the selected provenances of Sandal in southern India.
Zoo’s Print Journal 22(7): 2765–2767; http://dx.doi.org/10.11609/
JoTT.ZPJ.1593.2765-7
bramanian .A.
. India-A Lifescape, Dragonflies of India - A
Field Guide. Vigyan Prasar, India Offset Press, New Delhi, 118pp.
bramanian .A.
. Endemic odonates of the Western Ghats:
Habitat distribution and Conservation, pp. 257–271. In: Tyagi, B.K.
(ed.). Odonata-Biology of Dragonflies. Scientific Publishers, Jodhpur,
India.
bramanian .A.
. Dragonflies and Damselflies of Peninsular
India - A Field Guide. Vigyan Prasar, Noida, India, 168pp.
bramanian .A.
. A Checklist of Odonata of India. Zoological
Survey of India, Kolkata, 31pp.
bramanian .A. . a assery M. . air
. Chapter 5. The
status and distribution of dragonflies and damselflies (Odonata) of
the Western Ghats, pp. 63–86. In: Molur, S., K.G. Smith, B.A. Daniel
& W.R.T. Darwall (comp.). The Status and Distribution of Freshwater
Biodiversity in the Western Ghats, India. Cambridge, UK; IUCN
Gland, Switzerland; and Zoo Outreach Organisation, Coimbatore,
India, 117+vi
bramanian .A.
. . i arama rishnan
. Conservation of
Odonate fauna in Western Ghats, pp. 11–22. In: Sanjayan, K.P., V.
Mahalingam & M.C. Muralirangan (eds.). Vistas of Entomological
Research for The New Millennium. G.S. Gill Research Institute,
Chennai.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
6131
Adarsh et al.
© C.K. Adarsh
© C.K. Adarsh
Odonates in Kerala Agricultural University campus
ma e . Lestes praemorsus
ma e . Lestes elatus
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
ma e . Vestalis apicalis
ma e . Libellago lineata
ma e . Dysphaea ethela
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
ma e . Vestalis gracilis
ma e . Prodasineura er calis
ma e . Agriocnemis pieris
ma e
6132
. Agriocnemis pygmaea
ma e
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
ma e . Copera marginipes
. Ceriagrion cerinorubellum
ma e
. Ceriagrion coromandelianum
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
Adarsh et al.
© C.K. Adarsh
© C.K. Adarsh
Odonates in Kerala Agricultural University campus
Image 13. Ceriagrion rubiae
Image 15. Pseudagrion microcephalum
© C.K. Adarsh
Image 14. Ischnura aurora
Image 18. Gynacantha bayadera
Image 16. Pseudagrion rubriceps
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
Image 17. Anax immaculifrons
Image 21. Ictinogomphus rapax
Image 19. Gynacantha dravida
© C.K. Adarsh
© C.K. Adarsh
Image 20. Heliogomphus promelas
Image 22. Paragomphus lineatus
© C.K. Adarsh
Image 24. Acisoma panorpoides
Image 23. Epophthalmia vittata
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
6133
Adarsh et al.
ma e
© C.K. Adarsh
© C.K. Adarsh
Odonates in Kerala Agricultural University campus
. rac di la c al ea
. rac di la so rina
. Brachythemis contaminata
© C.K. Adarsh
ma e
. Diplacodes trivialis
ma e
© C.K. Adarsh
ma e
. a recis a asia ca
ma e
. Neurothemis tullia female
. ndo e is carna ca
© C.K. Adarsh
ma e
. Diplacodes nebulosa
. Bradinopyga geminata
© C.K. Adarsh
ma e
ma e
© C.K. Adarsh
ma e
male
© C.K. Adarsh
© C.K. Adarsh
ma e
ma e . Brachythemis contaminata
female
. euro e is ul ia female
ma e
. euro e is ul ia male
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
© C.K. Adarsh
Image 39. Orthetrum glaucum
© C.K. Adarsh
Image 37. Neurothemis tullia(male)
© C.K. Adarsh
Adarsh et al.
© C.K. Adarsh
Odonates in Kerala Agricultural University campus
image 42. Orthetrum sabina
Image 38. Orthetrum chrysis
Image 40. Orthetrum luzonicum
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
image 41. Orthetrum pruinosum
Image 43. Pantala flavescens
Image 45. Rhodothemis rufa (male )
© C.K. Adarsh
Image 46. Rhodothemis rufa (female )
© C.K. Adarsh
© C.K. Adarsh
Image 44. Potamarcha congener
Image 48. Rhyothemis variegata (male)
image 47. Rhyothemis variegata (female)
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
6135
Odonates in Kerala Agricultural University campus
Adarsh et al.
© C.K. Adarsh
Image 52. Tholymis tillarga (male)
© C.K. Adarsh
Image 50. Tetrathemis platyptera
Image 49. Trithemis aurora
Image 53. Tramea limbata
Image 51. Tholymis tillarga (female)
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
Image 55. Urothemis signata (male)
© C.K. Adarsh
© C.K. Adarsh
Image 54. Trithemis pallidinervis
Image 56. Zyxomma petiolatum
6136
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
Adarsh et al.
A
endi
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
© C.K. Adarsh
Odonates in Kerala Agricultural University campus
. ma es of the ater bodies in the st dy area.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6127–6137
hreatened a a
6137
o rnal of hreatened a a
Namdapha Gliding Squirrel
Biswamoyopterus biswasi is one of
the Critically Endangered (Molur
2008) and endemic gliding squirrels
ISSN
Online 0974–7907
found in India. It is thought to
Print 0974–7893
inhabit the Mesua ferrea jungles
P A
bordering the Noa-Dihing River of
Namdapha National Park (NNP),
Arunachal Pradesh, India. During our survey on gliding
squirrel diversity in NNP, we confirmed the presence
of the Red Giant Gliding Squirrel Petaurista petaurista
(RGGS) and Parti-colored Gliding Squirrel Hylopetes
alboniger through spotlighting and a specimen of
the Spotted Giant Gliding Squirrel Petaurista elegans
collected from this region is preserved at ZSI, Kolkata
(specimen named as P. sybilla, Collection No. NM/30).
However, we did not encounter Namdapha Gliding
Squirrel (NGS) during our multiple surveys (6 field
sessions including 120 survey nights). Its discovery in
the 1980s (Saha 1981; De et al. 2006) is the only time
when the species was originally studied (dead specimen)
after which it has neither been sighted nor documented.
However, many tourists, forest department personnel
and researchers claim the documentation of the
species, but no published record is available on the
sightings of the species elsewhere. Before the start of
our initial survey on gliding squirrels of NNP, we tried to
gather information on the possible distribution of NGS
through forest personnel (rangers, forest watchers and
guards). However, during our multiple surveys in NNP
we recorded RGGS in those areas which are thought to
be occupied by NGS according to the forest personnel.
.threatenedta a.or
ly
P
M
P
A
P
. M rali rishna
A adhesh
mar
1,2
Wildlife Resource & Conservation Lab, Department of Forestry,
North Eastern Regional Institute of Science and Technology (NERIST),
Nirjuli, Arunachal Pradesh 791109, India
1
murali7murali gmail.com (corresponding author),
2
tpileatus gmail.com
We interacted with many of the tourists (n 21), and
researchers (n 9) who have visited NNP and from those
interactions, we realised that the majority of them
mistake RGGS as NGS. So, based on those interactions,
we concluded many reasons behind the ignorance of
people towards the identification of the species, some
of which are listed below:
(i) S.S. Saha described the species NGS in the year
1981 and the data regarding the species pelage colour
and original photographs of it are documented only in
the paper published by the Bulletin of Zoological Survey
of India which is not available online because the paper
is often not in circulation (Image 1). Also, many other
papers which provide the description of NGS are not
often under circulation (Choudhury 2009). The holotype
is preserved at ZSI, Kolkata; no other voucher specimens
occur elsewhere (Image 2).
http://dx.doi.org/10.11609/JoTT.o3727.6138-41 | oo an urn:lsid:zoobank.org:pub:F7794CAB-B03F-431C-AFAA-B77D6BDD298F
ditor Anwaruddin Chowdhury, Guwahati, India.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3727 | Received 30 July 2013 | Final received 06 May 2014 | Finally accepted 08 July 2014
itation Krishna, C.M. & A. Kumar (2014). Why the Red Giant Gliding Squirrel Petaurista petaurista is often mistaken for the Namdapha Gliding Squirrel Biswamoyopterus biswasi (Mammalia: Rodentia: Sciuridae) in Namdapha National Park, Arunachal Pradesh, India. Journal of Threatened Taxa 6(8): 6138–6141; http://dx.doi.
org/10.11609/JoTT.o3727.6138-41
o yri ht © Krishna & Kumar 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
ndin The equipments for the project were funded by IdeaWild Grant, USA.
om etin nterest The authors declare no competing interests.
Ac no led ements We thank the Principal Chief Conservator of Forest (Wildlife & Biodiversity), Arunachal Pradesh and the Field Director and Research O cer
of Namdapha National Park for granting us permission to carry out the survey. Also, we thank the Director, Dr. Gaurav Sharma and Dr. Gopinathan Maheswaran
of ZSI, Kolkata for allowing us to study and photograph the gliding squirrel specimens. Kamalakaran, ZSI Kolkata is also thanked for guiding us through the gliding
squirrel specimens. We are grateful to IdeaWild Grant, USA for the equipment support. Lastly we thank Bironjay Basumatary, Erebo Chakma, Tinku Chakma and
Sambu Chakma for their assistance during the surveys. Also, we thank the reviewers for necessary comments that helped in building up this manuscript.
The elusive Namdapha Gliding Squirrel
Krishna & Kumar
(ii) Many websites on the internet often mistakenly
present RGGS as NGS even Arkive sections included
other gliding squirrel (possibly Hodgson’s Gliding
Squirrel) stating it to be NGS, which was later removed
(Arkive 2010).
(iii) RGGS has nearly 5–10 subspecies and the
variation of the pelage colour is observed (Corbet & Hill
1992; Yu 2002) this could be one of the reasons behind
the incorrect identification of the species. Moreover,
both NGS and RGGS share similar habitats in NNP.
(iv) Endemism is the other reason behind the
thought. As NGS is thought to be endemic to NNP in the
state of Arunachal Pradesh, India (De et al. 2006) many
people have a perception that the gliding squirrel found
in NNP is NGS.
(v) The Indian mammal field guide by Menon (2003,
2014) though provides a photographic plate showing
the head portion of NGS, the poor picture quality could
be a reason for wrong identification of the same.
(vi) The specimen at Miao museum of Namdapha
National Park cum Tiger Reserve holds a specimen of
gliding squirrel named as NGS which actually is RGGS
(Image 3). It was corrected as RGGS only in early 2012
after our suggestion.
Thus, we thought to provide important information
regarding the morphological characters of NGS with
ma e . a b c ho in the head dorsal and entral ortion of amda ha lidin
irrel is a o o erus is asi
. . aha aha
yello circle sho in the hite colo red ear t s in
re a d ho in the ict re of ed iant lidin
irrel Pe auris a
petaurista
. M rali rishna
ma e . om arati e acco nt of the head ortion front side
A amda ha lidin
irrel hoto ra hed from
ol ata
ed iant lidin
irrel
. M rali rishna
ma e . om arati e acco nt of head ortion side ise .
A
ello
ortion hi hli htin the ear t s hoto ra hed
from
ol ata
Photo ra hed from
P M se m at
Miao .
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6138–6141
The elusive Namdapha Gliding Squirrel
Krishna & Kumar
able . om arison of mor holo ical characteristics of amda ha lidin
Mor holo ical characters
amda ha lidin
irrel and ed iant lidin
irrel is a o o erus is asi
irrel
ed iant lidin
irrel
Pe auris a e auris a candidula
1.
Total Body Length
1010mm
1150mm
2.
Head & Body
405mm
520mm
3.
Hind Foot
78mm
75–100 mm
4.
Tail
605mm
630mm
5.
Pelage Colour
Body above is morocco red grizzled with white
Body above is partially morocco red grizzled with white
dominated by greyish and blackish
6.
Crown Colour
Grey hair tipped with red
White intermixed with black hair
7.
Tail colour
parti-coloured and beyond the inter-femoral membrane
proximally pale smoky gray changing distally to vinaceous
rufous and then to clove brown coloured near tip
Greyish beyond the inter-femoral membrane and then to
black coloured near tip
8.
Facial markings
Red coloured circles around eyes and narrow black lines
forming nasal bridge
Prominent Red coloured circles intermixed with black
around eyes followed by black markings above the nose
and below the chin.
9.
Patagium
Ventral side washed with faint orange-rufous
Ventral side with white hair
10.
Edges of Patagium
Red colour
Ashy colour
11.
Ear tufts
Distinct White ear tufts with silvery white margins on the
posterior side but the anterior margins basally
No such ear tufts are present
12.
Ventral Body Colouration
White with hairs with pearly grey bases
Pure white coat
Of the 5-10 subspecies of Petaurista petaurista, P. p. candidula is the subspecies that is observed in Namdapha National Park.
ma e . om arati e acco nt of dorsal ortion. A
Miao .
hoto ra hed from
ma e . om arati e acco nt of entral ortion. A
Photo ra hed from
ol ata
ol ata
hoto ra hed from
an an
mar as
P M se m at
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6138–6141
The elusive Namdapha Gliding Squirrel
Krishna & Kumar
eferences
ma e . om arati e acco nt of tails. A
. M rali rishna
. . aha
aha
photographs from published works, which will help in
identification of the species (Images 4–6).
Morphological characteristics of Namdapha Gliding
Squirrel Biswamoyopterus biswasi as described by Saha
(1981), Choudhury (2009) and Thorington et al. (2012)
are presented in Table 1.
Of the above mentioned characteristics, the presence
of the white coloured ear tufts is the main differentiating
character of NGS from RGGS, also,the adult individual of
NGS is smaller in size when compared to RGGS (Total
Body length: 1150mm).
Ar i e
. http://www.arkive.org/namdapha-gliding-squirrel/
biswamoyopterus-biswasi/ Downloaded on 20 September 2010.
ho dh ry A.
. One more new species of Giant Flying Squirrel
of the Genus Petaurista Link, 1795 from Arunachal Pradesh in
north-east India. Ne sletter and Journal of the hino Foundation
for nature in NE India 8: 26–34.
orbet . .
. . ill
. The Mammals of the Indo-Malayan
egion A Systematic evie . Oxford University Press, Oxford,
United Kingdom, 307–314pp.
e . . A. . Mandal M. . hosh
. Mammals. Bulletin of
Zoological Survey of India. Fauna of Arunachal Pradesh, State Series
13(1): 21–68.
Menon .
. A Field uide to Indian ammals. Dorling
Kindersley (India) Pvt. Limited and Penguin Book India (P) Ltd, New
Delhi, 200pp.
Menon .
. Indian ammals A Field uide. Hachette India,
New Delhi, 528pp
Mol r .
. Biswamoyopterus biswasi. In: IUCN 2013. IUCN Red
List of Threatened Species. Version 2013.2. www.iucnredlist.org .
Downloaded on 06 May 2014.
aha . .
. A new genus and new species of flying squirrel
(Mammalia: Rodentia: Scuiridae) from northeast India. Bulletin of
Zoological Survey of India 4(3): 331–336.
horin ton . . r. . . o ro s i M.A. teele
. . ha on
. S uirrels of the orld. Johns Hopkins University Press,
Baltimore, Maryland, 459
.
. Systematics and biogeography of flying squirrels in
the eastern and the western trans-Himalayas. A dissertation
presented to the graduate school of the University of Florida in
partial fulfilment of the requirements for the degree of doctor of
philosophy. University of Florida.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6138–6141
hreatened a a
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
Zoological gardens display wild
animals for aesthetic, recreational,
educational and conservation
purposes (Varadharajan & Pythal
ISSN
Online 0974–7907
1999). One of their goals is to
Print 0974–7893
preserve rare and endangered
OPEN ACCESS
species and in many parts of the
world, parks and zoological gardens
play an important role in species conservation (Parsani
et al. 2001). In their natural habitat, wild animals
have large areas available to them. Their exposure to
parasitic infections is, therefore, fairly low and they have
consequently a low genetic resistance against parasitic
infections. When groups of these wild animals are kept
in confined spaces in zoological gardens, the problem
of parasitic infections can aggravate and pose a serious
threat to the animals, occasionally causing sudden local
fatalities (Muoria et al. 2005).
The occurrence of parasites in animals housed in
zoos varies according to the type of husbandry, parasite
prophylaxis and type of parasitic treatment. Usually,
captive animals in the zoo do not show alarming signs
of parasitism if deworming is carried out regularly
(Parsani et al. 2001). Zoological gardens however
are often located near city centers, where space is
limited and many captive animal species are housed in
close proximity to each other. Because of these space
limitations, animals in these facilities succumb more
frequently to parasitic infections, which can pose a
serious health threat (Hoberg et al. 2008). In addition
in captivity animals are often under considerable stress,
which further diminishes their resistance to parasitic
infections.
Most. Monjila Khatun 1, Nurjahan Begum 2,
Md. Abdullah Al Mamun 3, Md. Motahar Hussain
Mondal 4 & Md. Shakif-Ul-Azam 5
1,2,3,4
Department of Parasitology, Faculty of Veterinary Science,
Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
5
Scientific O cer, Dhaka Zoo, Mirpur, Dhaka, Bangladesh
1
vet kmonjila yahoo.com, 2 nurjahanpara yahoo.com,
3
mamun.dvm gmail.com (corresponding author),
4
mmhmondal yahoo.com, 5 shakif78 gmail.com
In Bangladesh, a few zoological gardens, safari parks
and eco parks have been established which act as an
important source of recreation for people of all ages.
Until this date only few detailed and comprehensive
studies have been conducted on the prevalence of the
gastrointestinal parasites in animals housed in these
facilities. Therefore, this study attempts to determine
the occurrence and prevalence of gastrointestinal
parasites in zoo animals at Rangpur Recreational Garden
and Zoo in Bangladesh.
Study period, site and animals: This study was
conducted during April and September 2011 at Rangpur
Recreational Garden and Zoo which is located at
Rangpur, in northern Bangladesh. It is the smaller
one of two government zoos and was established in
1991, comprises an area of 20.7 acres and houses a
total number of over 200 animals including mammals,
DOI: http://dx.doi.org/10.11609/JoTT.o3093.6142-7
Editor: Ulrike Streicher, Wildlife Veterinarian / Wildlife Management Consultant, Danang, Vietnam.
ate of
blication 26 July 2014 (online & print)
Manuscript details: Ms # o3093 | Received 30 July 2013 | Final received 06 May 2014 | Finally accepted 08 July 2014
itation Khatun, M.M., N. Begum, M.A.A. Mamun, M.M.H. Mondal & M. Shakif-Ul-Azam (2014). Coprological study of gastrointestinal parasites of captive animals
at Rangpur Recreational Garden and Zoo in Bangladesh. Journal of Threatened Taxa 6(8): 6142–6147; http://dx.doi.org/10.11609/JoTT.o3093.6142-7
Copyright: © Khatun et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
Funding: The study was funded jointly by the first author and the Department of Parasitology, Bangladesh Agricultural University,
Mymensingh, Bangladesh.
om etin nterest The authors declare no competing interests.
Acknowledgements: The authors are very grateful to the Deputy Curator of Rangpur Recreational Garden and Zoo for giving permission to collect fecal samples
from the zoo. Sincere gratitude also to the animal workers for their cordial support and technical assistance during the sample collection. Special thanks to the
Head, Department of Parasitology, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh for his kind assent to use the laboratory.
6142
astrointestinal arasites of ca ti e animals
Khatun et al.
Table 1. ist of sam led animals ith their scienti c name and feed i en at an
Common name
cienti c name
No. of animals
(male-female)
r ecreational arden and oo in an ladesh.
No of samples
collected
Feed
Indian Lion
Panthera leo persica
4(3-1)
4
Beef
Royal Bengal Tiger
Panthera tigris
2(1-1)
2
Beef
Hyena
Crocuta crocuta
1(1-0)
1
Beef
Python
Morelia spilota variegata
2(1-1)
2
Chicken
Indian Bear
Melursus ursinus
2(0-2)
2
Mixed boiled feed (Rice, milk, egg, banana,
seasonal vegetables etc.)
acaca mulatta
6(5-1)
6
Fruits, bread, seasonal vegetables, Cereal grains.
Rhesus Monkey
Olive Baboon
Papiocyno cephalus anubis
Spotted Deer
Axis axis
Sambar Deer
Cervus unicolor
2(1-1)
2
Fruits, bread, seasonal vegetables, Cereal grains.
36
23
Grass, cereal grains
1(1-0)
1
Grass, cereal grains
Water Buck
Kobus ellipsiprymnus
1(1-0)
1
Grass, cereal grains
Hippopotamus
Hippopotamus amphibius
1(1-0)
1
Grass, cereal grains
reptiles and birds. The study included the carnivores,
nonhuman primates and herbivores housed at the zoo.
A total of 45 samples were collected. The samples were
collected once from each animal listed in Table 1.
Sampling and parasitological examination: With the
assistance of the animal caretakers individual fresh fecal
samples were collected. Because of the small number
of animals, it was possible to associate each sample with
a known individual. In the case of the tigers and lions,
the individual animal was kept separately overnight
and with the help of animal caretaker the sample was
collected the next morning. For spotted deer, individual
samples were collected immediately after defecation
when the deer were supplied with feed. Attention was
paid when a deer defecated, then its sex was identified
and the fresh fecal sample was collected from the
floor. Sample collection from hippopotamus, sambar,
water buck and hyena was easy due to presence of
only one animal in each cage. In python, the samples
were collected separately from the animals bedding.
It was easy because there were only two pythons in a
large cage that were far from each other. In the case
of the nonhuman primates (Rhesus Macaque and Olive
Baboon) the individual sample was collected by keeping
the animal separate the previous day with the help of
caretaker.
The fecal sample was placed in a polythene bag
containing 10% formalin and the sample was marked
according to species and sex, and finally examined in the
laboratory. The ova, cysts, oocyst and larvae of different
parasites were identified according to the morphology
and quantitative estimation was done by employing
Stoll’s ova counting technique (Soulsby 1982).
Results: A total of 45 fecal samples of different animals
were examined for the presence of gastrointestinal
parasites. The overall prevalence of parasitic infection
was 60% (27/45) with 35.6% (16/45) of helminth
infections and 24% (11/45) of protozoic infections.
Results indicated that helminths infections were more
common than protozoic infections in carnivores and
herbivores, whereas in primates protozoic infection was
more common than helminth infection (Table 2).
At least one intestinal parasite was identified in the
fecal sample of each animal except in the bears, pythons,
the water buck and olive baboons. Mixed infection was
observed in three species, including Rhesus Monkey
(Trichuris sp. + Balantidium coli), deer (Strongyloides
sp. + Coccidia) and lion (Toxascaris leonina + Spirometra
sp.) (Table 4). 72.7% (8/11) of the carnivores were
found positive for gastrointestinal parasites of which
9.1% (1/11) were protozoa, whereas 63.6% (7/11) were
helminths (Table 2). Parasites identified in carnivores
comprised Toxascaris leonina, Spirometra sp., Toxocara
cati and Balantidium coli. Lions were found infected
with Toxascaris leonina (100%, 4/4) and Spirometra sp.
(25%, 1/4). Tigers were found infected with Toxocara
cati (100%, 2/2) (Table 3).
Of the herbivores 50% (13/26) of the animals were
positive for gastrointestinal parasites of which 19.2%
(5/26) were protozoa and 30.8% (8/26) were helminths
(Table 2). Parasites identified in herbivores were Fasciola
sp., Moniezia benedeni, Strongyloides sp., Dictyocaulus
sp., stomach worm, Coccidia and Balantidium coli.
In the primates, 75% (6/8) of the animals were
positive for gastrointestinal parasites of which 62.5%
(5/8) were protozoa and 12.5% (1/8) were helminths
(Table 2). The species identified were Balantidium coli
and Trichuris sp. and were found in Rhesus Macaque.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
6143
astrointestinal arasites of ca ti e animals
Table 2.
Khatun et al.
erall re alence of astrointestinal arasites in ca ti e animals at an
Types of
animal
o. of ositi e sam le
r ecreational arden and oo.
Pre alence
No. of sample
examined
Protozoa
Helminth
Total
Protozoa
Helminth
Total
Carnivores
11
1
7
8
9.1
63.6
72.7
Herbivores
26
5
8
13
19.2
30.8
50.0
Primates
8
5
1
6
62.5
12.5
75.0
Total
45
11
16
27
24.4
35.5
60.0
Table 3. Pre alence of di erent astrointestinal arasites in ca ti e
animals at an
r ecreational arden and oo.
Name of the
animal
Name of the parasite
o. of ositi e
case (No of
sample)
Pre alence
Toxascaris leonina
4(4)
100
Spirometra sp.
1(4)
25
Royal Bengal
Tiger
Toxocara cati
2(2)
100
Hyena
Balantidium coli
1(1)
100
Indian Lion
Spotted Deer
Sambar Deer
Rhesus Monkey
Hippopotamus
Fasciola sp.
3(23)
13
Moniezia benedeni
1(23)
4.4
Strongyloides sp.
2 (23)
8.7
Dictyocaulus sp.
1(23)
4.4
Coccidia
3(23)
13
Stomach worm
1(23)
4.4
Balantidium coli
1(1)
100
Balantidium coli
5(6)
83.3
Trichuris sp.
1(6)
16.7
Balantidium coli
1(1)
100
In this study, the sex related prevalence could only
been assessed in deer as only in this species a suitable
sample size was available. Here the prevalence of
gastrointestinal parasites was higher in females (50%)
than in males (33.3%) (Table 5).
As not enough samples of each species were
available mean egg per gram of feces (EPG), ova per
gram of feces (OPG), cyst per gram of feces (CPG) and
larvae per gram of feces (LPG) were not calculated. So
the results presented in Table 6 simply show the lowest
and the highest numbers found in any sample. The
highest infection rate was found for Balantidium coli
with 1400 CPG in Rhesus monkey, followed by a rate
of 700 CPG in hippopotamus, and a rate of 300 CPG in
hyena and sambar deer.
Discussion: It has to be pointed out that the number
of animals in the study was very low and the results,
though they are interesting, are statistically irrelevant
and rather anecdotical. 60% of the animals at Rangpur
6144
Table 4. Mi ed infection recorded at an
and oo.
r ecreational arden
Name of the
animal
Name of the parasites
No. of cases
(N = 45)
Lion
Toxascaris leonina + Spirometra sp.
Deer
Strongyloides sp. + Coccidia
1
Rhesus Monkey
Trichuris sp. + Balantidium coli
1
1
Recreational Garden and Zoo were found positive for
gastrointestinal parasites. Other authors reported
similar (Parsani et al. 2001), higher (Opara et al. 2010,
Corden et al. 2008) or lower prevalences (Chakraborty
& Islam 1996; Lim et al. 2008), but prevalence always
ranged between 40.4 and 76.6%.
In all animals, except primates, the prevalence of
helminth infections was higher than the prevalence
of protozoic infections, an observation also confirmed
in other studies (Varadharajan & Kandasamy 2000;
Parsani et al. 2001). The high prevalence of helminths
encountered in the survey can be explained by the
favorable climatic conditions, which support prolonged
survival of infectious nematode larvae. The finding of
mixed infections might be due to presence of animals
of all age groups in the same cages, the feeding
management and improper disposal of feces.
In the present study 72.7% of the carnivores were
found positive for gastrointestinal parasites. Lower
(50%) and higher (97.3% and 89.3%) infection rates
were found by other authors (Muller-Graf 1995; Lim et
al. 2008). The main parasite found in carnivores were
tapeworm Spirometra sp. It has been stated a long time
ago that tapeworms were common among zoo animals
(Chauhan et al. 1973). Spirometra sp. however, though
the most common parasite in wild lions (Barutzki et al.
1985; Ghoshal et al. 1988), has not been reported in
zoo lions until 1995 (Muller-Graf 1995). Occurrence
of Spirometra depends on feeding management and
availability of intermediate hosts in the corresponding
areas. Two intermediate hosts are required to complete
the life cycle of Spirometra sp. The first intermediate
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
astrointestinal arasites of ca ti e animals
Khatun et al.
Table 5. e related re alence of di erent arasites in ca ti e deer
at an
r ecreational arden and oo
Sex
No. of
sample
examined
Male
9
Female
Total
14
s lar ae oocyst
of parasite
No. of
ositi e
cases
Pre alence
Fasciola sp.
1
11.1
Moniezia benedeni
1
11.1
Strongyloides sp.
1
11.1
Coccidia
1
11.1
Subtotal*
3*
33.3
Fasciola sp.
2
14.3
Strongyloides sp.
1
7.1
Dictyocaulus sp.
1
7.1
Stomach worm
1
7.1
Coccidia sp.
2
14.3
Subtotal
7
50.0
10
43.5
23
*= Total no. of animals affected is less than the summation of individual
infection because same animal was infected by more than one type of
parasites.
Heat destroys most of the ova or cyst of gastrointestinal
parasites and might be the reason for absence of
gastrointestinal parasites in bear in our study.
In this study 43.5% of the spotted deer were positive
for gastrointestinal parasites, which is lower than the
prevalence recorded by Kanungo et al. 2010 (75%). 13%
of the spotted deer were found positive for Fasciola
sp. This is lower than the rate of Fasciola sp. in deer
recorded by Kanungo et al. (2010) at Dhaka Zoo (20%)
and at Dulahazara Safari Park (19.1%). This difference
might be due to location of animal cages, availability
of intermediate hosts near the cages and the source of
feeds. The occurrence of Fasciola sp. infection in Dhaka
and Dulahazara was suspected to be connected with
mud snails that live on the edges of the drains and act
as intermediate hosts. Most of the deer cages at Dhaka
zoo are located near the lake of the zoo. Moreover,
the grass and leaves supplied to the deer are collected
outside of the zoo and might be contaminated with
Table 6. ntensity of o a cyst oocyst lar ae of di erent arasites in ca ti e animals at an
Name of the
animal
Indian Lion
No. of sample
examined
4
a cyst lar ae of arasite
o. of ositi e cases
(Male, Female)
r ecreational arden and oo.
ntensity of infection
P
P
P P
an es
Toxascaris leonine
4(3,1)
200–400
Spirometra sp.
1(0,1)
300
Royal Bengal Tiger
2
Toxocara cati
2(1,1)
300–400
Hyena
1
Balantidium coli
1(1,0)
300
Spotted Deer
23
Fasciola sp.
3(1,2)
200–300
Moniezia benedeni
1(1,0)
200
Strongyloides sp.
2(1,1)
100–200
Dictyocaulus sp.
1(0,1)
200
Coccidia
3(1,2)
300–400
Stomach worm
1(0,1)
300
Balantidium coli
5(4,1)
300–1400
Rhesus Monkey
6
Trichuris sp.
1(0,1)
100
Sambar Deer
1
Balantidium coli
1(1,0)
300
Hippopotamus
1
Balantidium coli
1(1,0)
700
hosts are crustaceans and snakes; birds, mammals etc.
are the second intermediate host (Soulsby 1982). So,
the presence of Spirometra sp. in the lion of Rangpur
Recreational Garden and Zoo might be due to ingestion
of contaminated beef.
In this study, no gastrointestinal parasite was
recorded in bear. This might be due to the feeding
management, deworming and sample size. Bears are
provided with a mixture of properly boiled ingredients.
metacercaria of trematodes (Kanungo et al. 2010). But
at Rangpur Zoo the chance of contamination is low as
the deer enclosures are located far from the lake of the
zoo and the grass supplied to the deer is cultivated at
the zoo.
In sambar only Balantidium coli was recorded, which
was different from other studies (Singh et al. 2009),
which found a large number of gastrointestinal parasites
including strongyles, Strongyloides sp., Coccidia, Fasciola
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
6145
astrointestinal arasites of ca ti e animals
Khatun et al.
sp., Amphistomes and Trichuris sp. This difference
might be due to the number of samples examined and
the housing and feeding management of the zoo. Only
one sambar is kept at Rangpur Recreational Garden
and it has been reared in captivity and the chance
for contamination from another individual and the
environment was low. Singh’s findings (2009) are made
in a group of sambar in a free range area at Mechendra
Choudhry Park, where the animals take to water readily
and swim with the body submerged, which might expose
them to infectiuos stages of parasites leading to higher
parasitic prevalence in the species. Moreover, the
moderate temperature range and the higher humidity
at the park lead to the formation of a permanent mud
area favorable to the survival of eggs and free-living
stages of parasites (Singh et al. 2009).
Among the primates 75% animals were positive for
gastrointestinal parasite infection. This result is much
lower than the prevalence of 88.7% reported by Mutani
et al. (2003), but higher than prevalences recorded by
Lim et al. (2008) (54.5%) and Stuart et al. (1990) (48%).
In accordance with other studies (Gomez et al. 1996;
Leveck et al. 2007; Lim et al. 2008) we found a higher
prevalence of protozoa (62.5%) than of helminths
(12.5%) in this animal group. The occurrence of these
parasites can be explained by the simplicity of their life
cycle, the low infective dose, the short prepatent period
and ability to survive in the environment. Balantidium
coli, which was commonly found in our study, has a wide
host range and possesses a simple direct life cycle and its
occurrence in primates has been previously confirmed
by Lim et al. (2008) and Gomez et al. (2000). Trichuris
sp., which was found in the primates in this study, has
been found by many authors (Lim et al. 2008; Singh et al.
2009) and is assumed to be the most common helminth
in primates (Corden et al. 2008).
The present study did not find gastrointestinal
parasites in Olive Baboon. Other studies however
found baboons usually infected with various helminths
(Nasher 1988; Murray et al. 2000; Mutani et al. 2003).
The fact that we did not find any parasites might be the
result of the number of samples, the animals’ immune
status and health condition, the deworming regime,
hygienic management and low density of the animals in
the enclosure.
In our study, sex related prevalence was only
assessed in deer, as only here a suitable sample size
was available. Here the prevalence of gastrointestinal
parasites was higher in females (50%) than in males
(33.3%). Although, the cause of the higher parasitic
infection rate in females is not known, it can be
6146
hypothesized that pregnancy, lack of feed supplements
during gestation and lactation, hormonal influences and
stress factors during gestation, parturition and lactation
may lead to an increased susceptibility for parasites.
Llyod (1983) reported higher level of prolactin and
progesterone hormones make an individual more
susceptible to any infection.
Conclusion: This is the first documentation of
gastrointestinal parasites of captive animals at Rangpur
Recreational Garden and Zoo. The high prevalence of
these parasites emphasizes the importance of controlling
these parasites in order to safeguard the health of the
housed animals and of humans working with these
animals. More studies of parasitic infections are
essential to understand the epidemiology of parasitism
and also to better prevent parasitic infections.
eferences
Barutzki, D., M.A. Hasslinger, K. Schmid & Wiesner (1985).
Situationsanalysez um Endoparasitenbefallbe i Zootieren. T ierarztliche mschau 40: 953–961.
Chakraborty, A. & S. Islam (1996). A survey of gastrointestinal parasitic
infection in some free ranging herbivores in the Kaziranga National
Park. Zoos’ Print 11(3): 3–5.
Chauhan, P.P. . . . hatia . . Arora . . A ra al . . Ahl alia
(1973). A preliminary survey of parasitic infections among mammals
and birds at Lucknow and Delhi Zoo. Indian Journal of Animal
Science 43(2): 163–168.
orden P. . . Prados A. omero M. . anche M. Pontes A.
s na M. . osales
. Intestinal parasitism in the animals
of the zoological garden Pen a Escrita’’ (Almun ecar, Spain).
Veterinary Parasitology 156: 302–309; http://dx.doi.org/10.1016/j.
vetpar.2008.05.023
hoshal . . . . ar
. . Misara lia
. . ain
. Helminth
parasites in zoo animals of Kamla Nehru Park, Indore (MP). Livestock
Adviser 13: 34–36.
ome M. . . orres M. racenea . Montoli C. Feliu, A.
Monleon, M.J. Fernadez & C. Ensenanat (1996). Intestinal
parasitism protozoa and helminthes in primates at the Barcelona
Zoo. Journal of edical Primatology 25: 419–423; http://dx.doi.
org/10.1111/j.1600-0684.1996.tb00038.x
ome M. . . orres M. racenea M. . ernade
M. . on ales
(2000). Further report on Cryptosporidium in Barcelona zoo
mammals. Parasitology Research 86: 318–323.
ober
.P. A.A. ocan
. . ic ard
. Gastro-intestinal
strongyles in wild ruminants, pp 193–227. In: Samuel W.M., M.J.
Pybus & A.A. Kocan (eds.). Parasitic Diseases of ild ammals nd Edition. Manson Publishing, London, UK, 559pp; http://dx.doi.
org/10.1002/9780470377000
an n o . A. as .M. as
ha if l A am
. Prevalence
of gastro-intestinal helminthiasis in captive deer of Bangladesh.
ayamba Journal of Animal Science : 42–45.
e ec
. P. . . orny . ercammen
. ereronysse
.
Gastrointestinal protozoa in non- human primates of four zoological
gardens in Belgium. Veterinary Parasitology 148: 236–246; http://
dx.doi.org/10.1016/j.vetpar.2007.06.020
im .A. . .
i . h ri M. ohela
. . . Mat
.
Intestinal parasites in various animals at a zoo in Malaysia.
Veterinary Parasitology 157: 154–159; http://dx.doi.org/10.1016/j.
vetpar.2008.07.015
Llyod (1983). Effects of pregnancy and lactation upon infection.
eterinary Immunopathology 4: 153–176.
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
astrointestinal arasites of ca ti e animals
M ller raf . .
. A coprological survey of intestinal parasites
of Wild Lions (Panthera leo) in the Serengeti and the Ngorongoro
crater. The Journal of Parasitology 81(5): 812–814.
M oria P. . P. M r thi . benstein . .
e
. M nene
(2005). Cross-sectional survey of gastro-intestinal parasites of
Grevy’s zebras in southern Samburu, Kenya. African Journal
of Ecology 43: 392–395; http://dx.doi.org/10.1111/j.13652028.2005.00588.x
M rray . . tem . o drea
. oodall
. Intestinal
parasites of Baboons (Papio cynocephalus anubis) and Chimpanzees
(Pan troglodytes) in Gombe national park. Journal of Zoo and
ildlife edicine 31: 176–178.
M tani A. . amara
. abriel
. A preliminary investigation
on the gastrointestinal helminths of the Barbados green monkey,
Cercopithecus aethiops sabaeus. evista do Institute de edicina
tropical SaoPaulo 45(4): 193–195.
Nasher, A.K. (1988). Zoonotic parasite infections of the Arabian Sacred
Baboon, Papio hamadryas arabicus Thomas, in the Asir Province,
Saud Arabia. Annals de Parasitologie humaine et comparee 63:
448–454.
Opara, M.N., C.T. Osuji & J.A. Opara (2010). Gastrointestinal
parasitism in captive animals at the zoological garden, Nekede
Owerri, southeast Nigeria. Report and Opinion 2(5): 21–28.
Khatun et al.
Parsani . . . . Momin M. . Maradin
. eer
. A survey
of gastrointestinal parasites of captive animals at Rajkot munipical
corporation zoo, Rajkot, Gujarat. Zoos’ Print Journal 16(10): 604–
606; http://dx.doi.org/10.11609/JoTT.ZPJ.16.10.604-6
in h P. . . M.P.
ta . harma
. . harma
.
Epidemiology and chemotherapy of parasitic infections in wild
omnivores in the Mahendra Chaudhary Zoological Park, Chhat
Bir, Punjab. Journal of Threatened Texa 1(1): 62–64; http://dx.doi.
org/10.11609/JoTT.o1767a.62-4
Soulsby, E.J.L. (1982). Helminths, Arthopods and Proto oa of
Domesticated Animals - th Edition. Bailliere and Tindal, London,
809pp.
t art M. . . . reens an . . lander M. . lar
.A
coprological survey of parasites of wild mantled Howling Monkeys
(Alouatta paplliata palliata). Journal of ildlife Disease 26: 547–
549; http://dx.doi.org/10.7589/0090-3558-26.4.547
aradhara an A. A. andasamy
A survey of gastro-intestinal
parasites of wild animals in captivity in the V.O.C. Park and Mini
Zoo, Coimbatore. Zoos’ Print Journal 15(5): 257–258; http://dx.doi.
org/10.11609/JoTT.ZPJ.15.5.257-8
aradhara an A.
. Pythal
. A preliminary investigation
on the parasites of wild animals at the zoological garden,
Thiruvananthapuram, Kerala. Zoos’ Print Journal 14(12): 159–164;
http://dx.doi.org/10.11609/JoTT.ZPJ.14.12.159-64
Threatened Taxa
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6142–6147
6147
Journal of Threatened Taxa |
The Asian Elephant Elephas
maximus Linnaeus, 1758 is listed as
an Endangered species (Choudhury
et al. 2008). The Sri Lankan sub
ISSN
Online 0974–7907
population of Asian Elephant is also
Print 0974–7893
listed as a Nationally Endangered
P A
species (MOE 2012). In addition
to its wild population, Sri Lanka
currently maintains a captive population of elephants
for various purposes including exhibition, work and
for cultural pageants (Canon & Davis 1995). Almost all
captive elephants in Sri Lanka are caught from the wild
at a young age. However, little work has been done to
evaluate how different management conditions affect
the physiology of captive elephants. Captive animals,
including elephants, have shown a tendency towards
being stressed (Odgen et al. 1994; Elzanowsky & Sergiel
2006) and chronic stress has been shown to reflect in the
haematology of animals by the reduction of eosinophils
circulating in the blood stream as well as lowering of
basophil counts (Barret et al. 2010), lowering of total
white blood cell (WBC) counts (Sutherland et al. 2006)
and increase of blood glucose concentration (Randall
et al. 2001). The increase of the ratio of neutrophils to
lymphocytes has also been shown to be an indicator of
stress (Rossdale et al. 1982; Kock et al. 1999). Therefore
we studied the haematology of elephants from three
different captive conditions to compare how the
management methods affected the haematology of the
animals.
Materials and Methods: The study was conducted
from July to September 2012. The three study
sites chosen were the National Zoological Gardens
.threatenedta a.or
A
A
ly
M
P
Ruvinda Kasun de Mel
e a a eerthi eera oon 2
ani ase ara aya atnasooriya 3 Asho a an olla 4
Department of Zoology, University of Colombo, Colombo 3,
Sri Lanka
4
Department of Veterinary Clinical Sciences, University of Peradeniya,
Peradeniya, Sri Lanka
1
ruvinda demel hotmail.com (corresponding author), 2 devakaw
gmail.com, 3 wdr zoology.cmb.ac.lk, 4 adangolla gmail.com
1,2,3
(6051’25.51 N & 79052’25.62 E) (NZG), the Pinnawala
Elephant Orphanage (7018’7.19 N & 80023’13.60”E)
(PEO) and the Millennium Elephant Foundation
(7016’24.58 N & 80023’1.20 E) (MEF). The NZG animals
live a highly sedentary life as they are chained for the
most part of the day and intra specific behaviour is
limited. The PEO animals are allowed to engage in intra
specific behaviours and are taken for baths in the Maoya’ river one kilometer away. The animals at MEF are
used for elephant rides throughout the day within the
MEF premises and thus have an opportunity for intra
specific behaviours as well as exercise.
Four adult females were sampled at NZG while
only two animals each could be sampled from PEO
and MEF (two adult males and two adult females) due
to logistical constraints. All animals were sampled on
four consecutive days between 0800–0900 hr. In order
: http://dx.doi.org/10.11609/JoTT.o3761.6148-50
ditor Ulrike Streicher, Wildlife Veterinarian / Wildlife Management Consultant, Danang, Vietnam.
ate of
blication 26 July 2014 (online & print)
Man scri t details Ms # o3761 | Received 07 September 2013 | Final received 11 July 2014 | Finally accepted 13 July 2014
itation de Mel, R.K., D.K. Weerakoon, W.D. Ratnasooriya & A. Dangolla (2014). A comparative haematological analysis of Asian Elephants Elephas maximus
Linnaeus, 1758 (Mammalia: Proboscidea: Elephantidae) managed under different captive conditions in Sri Lanka. Journal of Threatened Taxa 6(8): 6148–6150;
http://dx.doi.org/10.11609/JoTT.o3761.6148-50
o yri ht © de Mel et al. 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction
and distribution by providing adequate credit to the authors and the source of publication.
ndin None.
om etin nterest The authors declare no competing interests.
Ac no led ements The authors wish to thank Ms. Dhammika Malsinghe (Asst. Director National Zoological Gardens), Dr. Nirmalie Pallewatte (Head, Dept. of
Zoology, Faculty of Science, University of Colombo), Mr. H. Jayakody (Dept. of Zoology, Faculty of Science, University of Colombo), Dr. D.K. Nanayakkara (Dept.
of Nuclear Medicine, Faculty of Medicine, University of Peradeniya) , Dr. Jagath Jayasekera (Vet. Surgeon, National Zoological Gardens), Dr. C. Rajapakse (Vet.
Surgeon, Pinnawala Elephant Orphanage)
Haematological analysis of Asian Elephants
de Mel et al.
to draw blood, the elephants were ordered to assume a
lateral recumbancy position by the mahout. Then, 5ml
of blood was drawn from an ear vein on the posterior
surface of the ear by a qualified veterinary surgeon
using an 18 gauge sterile hypodermic needle (Sumbow
medical instruments, Ningbo, China) and a 10ml
disposable syringe (Changzhou medical appliances,
Jiangsu province, China). This method has been used
by Ratnasooriya et al. (1995). The collected blood was
then transferred into an EDTA coated tube (APTACA
Canelli-Italy) and gently turned upside-down 2-3 times
to ensure the blood wouldn’t clot. Blood smears were
prepared to be used in the differential counts which
were performed under an oil immersion microscope
(Model cx21FS2, Olympus Corporation, Tokyo, Japan),
while white blood cells (WBC) and red blood cell (RBC)
were counted using an improved neubauer double cell
hemocytometer (Hawksley & Sons Ltd, Sussex, England)
following previously established procedures (Dacie &
Lewis 1997). Packed Cell Volume (PCV) was determined
using a Hematocrit centrifuge (Hawksley, England). The
values of PCV and RBC counts were used to compute the
mean corpuscular volumes (MCV).
Minitab version 15 was used to analyse the
results statistically. The Kruskal-Wallis test at a 95%
confidence interval followed by a post-hoc test of a
pair wise comparison using Mann-Whitney U test at a
95% confidence interval was conducted to determine
differences between groups.
Ethical clearance for the project was obtained
from the Institute of Biology Sri Lanka (Registration
number: ERCIOB101/05/12) while permission from
the Department of National Zoological Gardens was
obtained to use their animals in the research project.
Results: Table 1 shows the results for the various
haematological parameters.
The glucose levels of the elephants kept at MEF
were significantly higher than the glucose levels of the
animals kept at NZG and PEO (Kruskal-Waalis, H 6.88,
able . Means
s of aematolo ical arameters of the three st dy ro
/
3
ite
l
Mean
2d.f., P 0.032,). The total WBC counts of elephants kept
at NZG were shown to be significantly lower than WBC
counts of elephants kept at PEO and MEF v (KruskalWallis, H 21.92, 2d.f., P 0.000). The lymphocyte counts
of the animals kept at NZG were significantly lower
compared to those of the animals kept at PEO and MEF
(Kruskal-Wallis, H 16.40, 2d.f., P 0.00). The percentages
of monocytes differed significantly between the three
groups (Kruskal-Wallis, H 16.73, 2d.f., P 0.000) with
the animals kept at NZG showing the highest values
while the animals kept at MEF had the lowest values.
The PCV differed significantly and were higher in the
elephants kept at NZG and PEO than in the elephants
kept at MEF. The ratio of neutrophils to lymphocytes
were again shown to be significantly different (KruskalWallis, H 14.58, 2d.f., P 0.05) with the NZG value being
highest. No other haematological parameter showed a
significant difference between the three groups (KruskalWallis, P 0.05).
isc ssion All values of the haematological
parameters found in our study fall within the ranges
previously reported for captive Asian Elephants
(Ratnasooriya et al. 1990; Yathiraj et al. 1992). However,
the total WBC counts and lymphocyte counts were
significantly lower while the ratio of neutrophils to
lymphocytes was significantly higher in the elephants
from NZG. Decreases of lymphocytes have been seen
previously in animals under stress due to confinement
and sleep deprivation (Ferrante et al. 1998; Po nger &
Pickering 1992; Zager et al. 2007) and similar decreases
of WBC have been seen in animals which were stressed
due to overcrowding and heat (Sutherland et al.
2006). Elevated ratios of neutrophils to lymphocytes
have also been observed in stressed animals such as
horses (Rossdale et al. 1982), rhinos (Kock et al. 1999)
and rhesus monkeys (Morrow-Tesch et al. 1993). The
significantly elevated glucose levels of the animals kept
at MEF could be due to the fact that they receive food
material rich in glucose (fruits, sugar cane) between
/
6
l
Mean
s.
ndicates si ni cantly di erent arameters
e tro hils osino hils
%
%
Mean
Mean
aso hils
Mean
ym hocytes Monocytes
%
%
Mean
Mean
lood
l cose
mmol l
Mean
P
Mean
M
Mean
e t
y mh
Mean
5.02±0.32
3.42 0.32
49.05 3.16
5.34 2.03
1.56 0.98
40.05 1.95
3.32±1.17
4.7 0.51
36.63±5.65
107.4 16.07 1.22±0.12
P
8.17±0.77
3.87±0.58
44.5 6.30
4.11 2.98
1.6 1.40
46.51 6.59
2.38±1.17
4.61 0.35
36.12±1.81
94.81 13.06 0.99 0.29
M
7.76±0.66
3.21 0.44
40.51 1.19
3.46 0.52
1.1 0.29
53.3 1.46
0.79 0.47
5.37±0.63
28.83 4.07
1.13 7.90
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6148–6150
0.76±0.03
Haematological analysis of Asian Elephants
de Mel et al.
the walks. This would elevate the blood glucose levels,
which would be reflected in the tests. The reason for the
significantly lower monocyte counts found in elephants
kept at MEF and PEO in comparison to the counts found
in animals from NZG remains at the moment unknown.
The significantly reduced PCV values of the elephants
from MEF compared to the elephants from NZG and PEO
could be due to the fact that the elephants kept at MEF
have more opportunities to keep themselves hydrated.
Dehydration has been shown to be a promoting factor
of high PCV values (Maloiy & Boarer 1971). The
elephants from MEF during their many walks come
regularly to the waterway and this provides them with
ample opportunity to drink and sprinkle water on their
bodies. This may be important for elephants as they
are poor thermoregulators (Weissenbock et al. 2012).
Dehydration in the elephants kept at NZG could thus
be a result of comparatively limited access to water
and also be a factor that promotes a certain amount
of chronic stress. Due to logistical reasons the sample
size in this study is small and prevents us from drawing
conclusive remarks with confidence. However, based
on the haematological parameters assessed in this
study, we conclude that the elephants at NZG are under
slightly higher levels of stress compared to the elephants
at PEO and MEF. The assessment of Cortisol hormone
(de Mel et al. 2013a) and the assessment of stereotypic
behaviour (de Mel et al. 2013b) done using these same
animals simultaneously to this study indicates that the
NZG animals were at a higher level of chronic stress
comparatively. However, all elephants were apparently
healthy and therefore stress may not have affected them
to a significant level. Obviously more thorough and
comprehensive studies must be conducted and other
parameters to assess stress must be included before
definite conclusions can be drawn.
eferences
arret . . .M. arman . oitano
. . roo s eds.
.
Ganong’s Review of Medical Physiology - 23rd Edition. McGraw-Hill
Medical, 726pp.
anon P. P. a is
. ALIYA; Stories of the Elephants of Sri Lanka.
Airavata Press. Melbourne, 102pp.
ho dh ry A. . . . ho dh ry A. esai . .
c orth P. .
asa A. . . ohnsin h P. ernando . ed es M. na ardena
. rt . aranth A. ister . Menon . iddle A. bel
.
i ramanaya e
Asian le hant
ecialist ro
. Elephas maximus. The IUCN Red List of Threatened Species.
Version 2014.1. www.iucnredlist.org . Downloaded on 16 July
2014.
de Mel . . M. . .
mara A. an olla . ayase era . . .
anaya ara . a a a se . . atnasooriya
. . eera oon
a . An assessment of the stress levels of captive elephants
held under different management conditions using serum cortisol
levels. Book of Abstracts Peradeniya University Research Sessions.
17: 215.
de Mel . . . . eera oon
. . atnasooriya
b. A
comparison of Stereotypic Behaviour in Asian Elephants at Three
Different Institutions in Sri Lanka. Gajah 38: 25–29.
acie . .
.M. e is
. Practical Haematalogy. Longman
Singapore Publishers. Singapore, 41–55pp.
l ano s i A.
A. er iel
. Stereotypic behavoiur of a
female Asiatic Elephant (Elephas maximus) in a zoo. Journal of
Applied Animal Welfare Science 9: 223–232; http://www.dx.doi.
org/10.1207/s15327604jaws0903 4
errante . . anali . Ma ello M. er a P. acerdote .
Manfredi A. . Panerai
. Preliminary study on the effect of
size of individual stall on the behavioural and immune reactions of
dairy calves. Journal of Animal Feed Science 7(1): 29–36.
oc .A. . . . Miho . amb a . M an ia
. ai a a
.
Effects of translocation on hematologic parameters of free-ranging
Black Rhinoceros (Diceros bicornis michaeli) in Kenya. Journal of Zoo
and Wildlife Medicine 30: 389–396.
Maloiy .M. .
. . . oarer
. Response of the Somali
donkey to dehydration: hematological changes. American Journal of
Physiology 221(1): 37–41.
M
. The Taxonomy and Conservation Status of Mammals in
Sri Lanka, pp. 134–144. In: Weerakoon, D.K. & S. Wijesundara (eds.).
The National ed List
of Sri Lanka Conservation Status of the
Fauna and Flora. Ministry of Environment, Colombo, Sri Lanka.
Morro esch . . . . Mc lone
. . orman
. Consequences
of restraint stress on natural killer cell activity, behavior,
and hormone levels in Rhesus Macaques ( acaca mulatta).
Psychoneuroendocrinology 18: 383– 395.
den . . . . indb r
. . Ma le
. A preliminary study
of the effects of ecologically relevant sounds on the behaviour of
captive Lowland Gorillas. Applied Animal Behavouir Science (39):
163–176.
Po n er . .
A. . Pic erin
. The influence of social
interaction on the acclimation of Rainbow Trout, Oncorhynchus
mykiss (Walbaum) to chronic stress. Journal of Fish Biology 41(3):
435–447.
andall . . r ren
. rench
. Animal Physiology - 5th
Edition. W.H. Freeman and Company, New York, 338–339pp.
atnasooriya . . A. . . Amarasin he
. . odi ara
. Total
serum cholesterol levels of Sri Lankan Elephants (Elephas maximus
maximus). Ceylon Journal of Science 24(1): 11–16.
atnasooriya
. .
. . . ernando A.M. . . Manat n a
. . aldera
. . iyana e
.A. . Prema mara
.
Haematological values for adult Asian Elephants (Elephas maximus
maximus) at the Pinnawala Elephant Orphanage, Sri Lanka. Medical
Science Research 18: 899–902.
ossdale P. . P. . r e
. . . ash
. Changes in blood
neutrophil/lymphocyte ratio related to adrenocortical function in
the horse. Equine Veterinary Journal 14: 293–298.
therland M.A. . . ie am
. . odri e as
. . ala
Johnson
. Impacts of chronic stress and social status on
various physiological and performance measures in pigs of different
breeds. Journal of Animal Science 84(3): 588–596
eissenboc
.M.
. Arnold
.
f
. Taking the heat:
thermoregulation in Asian Elephants under different climatic
conditions. Journal of Comparative Physiology B 182(2): 311–319.
athira . P. . ho dh ri . . ao P. . eddy
. Clinicohaematological observations on Indian Elephant (Elephas maximus
indicus). Indian Veterinary Journal 69: 995–997.
a er A. M. . Anderson . . i . . Ant nes
.
.
Effects of acute and chronic sleep loss on immune modulation of
rats. American Journal of Phsiology- egulatory Integrative and
Comparative Physiology 293(1): 504–509.
Threatened Taxa
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6148–6150
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6151–6152
e ie of the boo
i er eser e
er ies and Moths of Pa e
llasa odandaramaiah
Indian Institute of Science Education and Research
Thiruvananthapuram (IISER-TVM), Sreekaryam, Thiruvananthapuram,
Kerala 695016, India
ullasa@iisertvm.ac.in
The book, a guide to the lepidopteran fauna of
Pakke Tiger Reserve in Arunachal Pradesh, northeastern
India, follows in the footsteps of the recently published
Butterflies of the Garo Hills’, the author list of which
includes Sanjay Sondhi and Krushnamegh Kunte.
Krushnamegh Kunte is one of the leading experts on
Indian butterflies and is well-known among butterfly
circles. Sanjay Sondhi is a keen naturalist, involved in
biodiversity documentation and conservation, especially
in northeastern India. He has a special interest in
Lepidoptera. The collective experience and expertise
of the two make them an excellent combination to
write such a book. After having largely relied on preIndependence literature to identify butterflies, I am glad
that Indian naturalists are finally taking to authoring
literature on our country’s butterflies.
The work is based almost entirely on circa 50 days
of field observations by the first author and a couple of
visits by the second author. It includes photographic
reports of 284 butterfly species, supplemented by
more photographs of similar looking species that are
likely to be found in the area. The authors themselves
state that the book is not a comprehensive guide to all
butterflies in the area. As far as moths are concerned,
the book only covers 83 moths in a concise 13 pages,
which form, for all practical purposes, an appendix to
butterflies. There are no species descriptions for moths;
each species features a photograph of a live specimen
with its latin name and wingspan.
Butterfly densities can vary tremendously not only
across seasons, but also over years. Categorization of
butterflies in a locality into common, rare and other
categories merely based on a total of about two months
of field work is bound to be tentative for many species.
The book would have been a worthwhile addition to
literature if it had been more comprehensive, i.e., based
on considerably more field data. As it stands now, I am
certain that a few more months of field work, and welldesigned bait-trapping, will add many more species to
the list.
Reviewing this book for a scientific journal, I must
state that from a purely academic
perspective, the book does not
add greatly to scientific literature.
I am mildly disappointed by the
premature’ publication of the
book. This is not a comprehensive
account of the diversity of
ISSN
Online 0974–7907
Print 0974–7893
OPEN ACCESS
er ies and Moths of Pa e i er eser e
Sanjay Sondhi and Krushnamegh Kunte
Date of publication: 2014
Publisher: Titli Trust (Dehradun) and Indian Foundation for
Butterflies (Bengaluru).
Pages: 202
Price: 500
butterflies in the region, and is certainly only a cursory
account of moth diversity. The inclusion of moths in
the title is rather misleading in so far as it covers but a
small fraction of the moth diversity in the locality. The
butterfly work would have been more aptly published as
DOI: http://dx.doi.org/10.11609/JoTT.o4046.6151-2
ate of blication 26 July 2014 (online & print)
6151
oo re ie
er ies and Moths of Pa e i er eser e
a paper in a journal such as JoTT. Data from several more
days of systematic light-trapping are needed before
the moth data qualifies to be published in a reputable
journal. As far as moths are concerned, I personally
had high expectations upon seeing the title, but ended
up being disappointed. Given the enormous diversity
of moths, and the complexity of their taxonomy, I am
afraid a beginner like me relying on the book may end
up wrongly identifying species based on photographic
similarity.
However, I gather the book is meant to promote
eco-tourism in the reserve. Indubitably a noble goal,
but I am unsure who the target users of the book are.
Butterfly and bird tourism is an industry waiting to
be tapped, especially in the context of improving the
livelihoods of local communities critical for conservation
in northeastern India. As a bird or butterfly enthusiast, I
would certainly prefer to carry a guide-book describing a
more or less comprehensive list of species in the region,
rather than an incomplete guide to a specific reserve,
even if the reserve in question were to be the only
place I plan to visit. Of course, we lack a comprehensive
guide to Arunachal Pradesh or northeastern Indian
butterflies, but a book like Isaac Kehimkar’s The book of
Indian butterflies’ is arguably more useful for a butterfly
watcher to purchase compared to a book that specializes
on a particular locality.
On the plus side, the lovely photographs are well
presented, including both dorsal and ventral surfaces
where needed, and some museum photographs that
are currently hard to access through literature. Hence,
even though this is not a comprehensive account, every
species described in the book can be unambiguously
identified provided one has usable photographs. This
is not necessarily the case with butterfly guides in India.
Many identifying characters of species presented in the
book are not found in other literature.
In my opinion, from the larger perspective of
conservation and promotion of natural history interest
in public, the need of the hour is to have comprehensive
field guides to our rich flora and fauna, both national
and regional. I urge authors embarking on authoring
such books to aim for more comprehensive and detailed
guides to their taxon of interest, and cover larger
geographic areas. That said, the proof is in the pudding.
If this book ends up promoting butterfly tourism in
and around Pakke and increases revenue for the local
communities, the book will be considered a success, and
will naturally spawn more such titles.
As a reviewer, I can recommend the book in many
cases, especially considering the quality of images and
the very reasonable price—Rs. 500. It is certainly a must
buy for wildlife enthusiasts living in the surroundings of
the reserve. As also for regular visitors to the reserve
who are keen butterfly watchers but unwilling to
deal with more complicated keys. If you want to chip
in towards conservation, like butterflies and enjoy
travelling, go ahead, buy a copy and visit Pakke!
Threatened Taxa
6152
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 July 2014 | 6(8): 6151–6152
Nancy van der Poorten, Toronto, Canada
Neelesh Dahanukar, IISER, Pune, Maharashtra, India
Nita Shah, Wildlife Institute of India, Dehra Dun, India
Okan K lk yl o lu, Abant Izzet Baysal University, Bolu, Turkey
P.M. Sureshan, Zoological Survey of India, Kozhikode, Kerala, India
P.O. Nameer, Kerala Agricultural University, Thrissur, Kerala, India
Pankaj Kumar, Kadoorie Farm and Botanic Garden Corporation, Hong Kong
Partha Pratim Bhattacharjee, Tripura University, Suryamaninagar, India
Peter Smetacek, Butterfly Research Centre, Bhimtal, India
Priya Davidar, Pondicherry University, Kalapet, Puducherry, India
R. Ramanibai, Guindy Campus, Chennai, Tamil Nadu, India
R. Varatharajan, Manipur University, Imphal, Manipur, India
R.K. Avasthi, Rohtak University, Haryana, India
R.K. Verma, Tropical Forest Research Institute, Jabalpur, India
R.M. Sharma, (Retd.) Scientist, Zoological Survey of India, Pune, India
Ragnar Kinzelbach, University of Rostock, Rostock, Germany
Rajah Jayapal, SACON, Coimbatore, Tamil Nadu, India
Rajan Amin, The Zoological Society of London, London, England
Rajeev Raghavan, St. Albert’s College, Kochi, Kerala, India
Rajiv S. Kalsi, M.L.N. College, Yamuna Nagar, Haryana, India
Raju Vyas, Vishwamitri River Project, Vadodara, India
Renkang Peng, Charles Darwin University, Darwin, Australia
Reuven Yosef, International Birding & Research Centre, Eilat, Israel
Richard Corlett, ishuangbanna Tropical Botanical Garden, Mengla, Yunnan, China
Richard Gallon, llandudno, North Wales, LL30 1UP
Richard Kiprono Mibey, Vice Chancellor, Moi University, Eldoret, Kenya
Robert D. Sluka, Chiltern Gateway Project, A Rocha UK, Southall, Middlesex, UK
Rory Dow, National Museum of natural History Naturalis, The Netherlands
S. Bhupathy, SACON, Coimbatore, Tamil Nadu, India
S. Jayakumar, Pondicherry University, Puducherry, India
Sanjay Molur, WILD, Coimbatore, Tamil Nadu, India
Shomita Mukherjee, SACON, Coimbatore, Tamil Nadu, India
Shonil Bhagwat, Open University and University of Oxford, UK
Stephen D. Nash, Scientific Illustrator, State University of New York, NY, USA
Sushil K. Dutta, Indian Institute of Science, Bengaluru, Karnataka, India
Tadashi Kawai, Wakkanai Fisheries Research Institute, Hokkaido, Japan
Topiltzin Contreras MacBeath, Universidad Autónoma del estado de Morelos, México
Ulrike Streicher, Wildlife Veterinarian, Danang, Vietnam
V. Irudayaraj, St. avier’s College, Palayamkottai, Tamil Nadu, India
V. Sampath Kumar, Royal Botanic Gardens, Kew, UK
V. Santharam, Rishi Valley Education Centre, Chittoor Dt., Andhra Pradesh, India
Vijayasankar Raman, University of Mississippi, USA
W. Vishwanath, Manipur University, Imphal, India
A. Biju Kumar, University of Kerala, Thiruvananthapuram, Kerala, India
A.K. Sreekala, JNTBGRI, Palode, Thiruvananthapuram, Kerala, India
Aniruddha Belsare, Columbia MO 65203, USA
Arun K. Pandey, University of Delhi, Delhi, India
Arun Kanagavel, WILD, Coimbatore, Tamil Nadu, India
Arun P. Singh, Rain Forest Research Institute (ICFRE), Jorhat India
Ashish D. Tiple, Vidyabharati College, Seloo, Wardha, Maharashtra, India
B. Ramesha, Kerala Agricultural University, Kasaragod District, Kerala, India
B.C. Suman, University of Horticulture and Forestry, Solan, India
Bakhtiar Effendi Yahya, Universiti Malaysia Sabah, Sabah, Malaysia
Basudev Tripathy, Zoological Survey of India, New Alipore, India
Bhargavi Srinivasulu, Osmania University, Hyderabad, India
Bulganin Mitra, Zoological Survey of India, New Alipore, West Bangal, India
Dhananjai Mohan, Vasant Vihar, Dehradun, India
Dilip Chetry, Gibbon Conservation Centre, Mariani, Jorhat, Assam, India
Dipankar Ghose, WWF-India, Max Muller Marg, New Delhi, INDIA
Dr. K.V. DheeRaj, Karnatak University, Dharwad, Karnataka, India
DR. Subhasis Panda, Darjeeling Govt. College, Darjeeling, West Bengal
G. Umapathy, Centre for Cellular and Molecular Biology, Hyderabad, India
G.K. Srivastava, Gomti Nagar, Lucknow, Uttar Pradesh, India
Gowri Mallapur, Madras Crocodile Bank Trust, Chennai, Tamil Nadu, India
H. Raghuram, The American College, Madurai, Tamil Nadu, India
Journal of Threatened Taxa is indexed/abstracted in
Bibliography of Systematic Mycology, Biological Abstracts,
BIOSIS Previews, CAB Abstracts, EBSCO, Google Scholar,
Index Copernicus, Index Fungorum, JournalSeek, National Academy of Agricultural Sciences, NewJour, OCLC
WorldCat, Stanford University Libraries, Virtual Library of
Biology, Zoological Records.
NAAS rating (India) 4.72
Hilloljyoti Singha, Assam University, Silchar, Silchar, Assam, India
Honnavalli N. Kumara, SACON, Anaikatty P.O., Coimbatore, Tamil Nadu, India
J.A. Johnson, Wildlife Institute of India, Dehra Dun, India
J.D. Marcus Knight, Velachery, Chennai, Tamil Nadu, India
Jayshree Vencatesan, Care Earth Trust, , Chennai, Tamil Nadu, India
Jigme Tshelthrim Wangyal, District Forest O ce, Trashigang, Bhutan
Justus Joshua, Green Future Foundation, Udaipur, Rajasthan, India
K. Karthigeyan, Botanical Survey of India, India
K. Praveen Karanth, Indian Institute of Science, Bengaluru, Karnataka, India
K.K. Vass, Chicago, USA
K.P. Dinesh, Zoological Survey of India, Chennai, Tamil Nadu, India
K.R. Sridhar, Mangalore University, Mangalore, Karnataka, India
K.S. Anoop Das, Mampad College, Malappuram District, Kerala, India
K.V. Dheeraj, Karnatak University, Dharwad, Karnataka, India
K.V. Gururaja, Indian Institute of Science, Bengaluru, Karanataka, India
Kalpana Pai, University of Pune, Pune, India
Karan Bahadur Shah, Natural History Museum, Tribhuvan University, India
Kareen Schnabel, NIWA, Wellington, New Zealand
Kashinath Bhattacharya, Visva-Bharati University, West Bengal, India
Krushnamegh Kunte, Harvard University, Cambridge, USA
L. Kosygin Singh, Zoological Survey of India, Hilltop, Odisha, India
L.J. Mendis Wickramasinghe, Herpetological Foundation of Sri Lanka, Sri Lanka
Laxman Prasad Poudyal, Shivapuri Nagarjun National Park, Nepal
M K Janarthanam, Goa University, Goa, India
M.K. Vasudeva Rao, Shiv Ranjani Housing Society, Pune, Maharashtra, India
Madhava Meegaskumbura, University of Peradeniya, Sri Lanka
Mandar Datar, Agharkar Research Institute, Pune, Maharashtra, India
Monsoon Jyoti Gogoi, Bokakhat, Assam, India
Muhamed Jafer Palot, Zoological Survey of India, Kozhikode, Kerala, India
N.A. Aravind Madhyastha, ATREE, Jakkur PO, Bangalore, India
N.D. Paria, Department of Botany, University of Calcutta, West Bengal, India
Nishith Dharaiya, HNG University, Patan, Gujarat, India
Nita Shah, Wildlife Institute of India, Dehra Dun, India
P. Lakshminarasimhan, Botanical Survey of India, Howrah, India
P. Venkateswara Prasanna, Botanical Survey of India, Andhra Pradesh, India
P. Venu, Botanical Survey of India, Howrah, India
P.S. Easa, Kerala Forest Research Institute, Peechi, India
Payal Molur, Coimbatore, Tamil Nadu, India
Purnendu Roy, Gabriel’s Wharf, London, UK
R. Mohanraju, Department of Ocean Studies & Marine Biology, Port Blair, India
R. Ramasubbu, Gandhigram Rural University, Dindigul, Tamil Nadu, India
R. Suresh Kumar, Wildlife Institute of India, Dehra Dun, India
Rauf Ali, Foundation for Ecological Research, Vazhakulam, Pondicherry, India
Renee M. Borges, Indian Institute of Science, Bengaluru, Karnataka, India
Rohan Pethiyagoda, Australian Museum, Australia
S. Ajmal Khan, Annamalai University, Parangipettai, India
S. Arularasan, Annamalai University, Parangipettai, India
S. Chellappa, Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil
S.K. Srivastava, Botanical Survey of India, Dehradun, Uttarakhand, India
S.R. Ganesh, Chennai Snake Park, Chennai, Tamil Nadu, India
Sanjay Sondhi, TITLI TRUST, Kalpavriksh, Dehradun, India
Seema Bhat, Independent Consultant, New Delhi, India
Sekar Raju, i’an Jiaotong-Liverpool University, Suzhou, China
Sery Ernest Gibedele Bi, Universit de Cocody Abidjan, C te d’Ivoire
Shrikant Jadhav, Zoological Survey of India, Akurdi, Pune, Maharashtra, India
Siby Philip, Nirmalagiri College, Nirmalagiri, Kannur, Kerala, India
Swapna Prabhu, Bombay Natural History Society, Mumbai, Maharashtra, India
Tulsi Subedi, Kathmandu, Nepal
Unmesh Katwate, Bombay Natural History Society, Mumbai, Maharashtra, India
Utpal Smart, University of Texas at Arlington, T 76019-0498, USA
V S. Ramachandran, Bharathiar University, Coimbatore, Tamil Nadu, India
V. Gokula, National College, Tiruchirappalli, Tamil Nadu, India
V.B. Hosagoudar, TBGRI, Palode, Kerala, India
V.C. Soni, Saurashtra University, Maharashtra, India
Vatsavaya S. Raju, Kakatiay University, Warangal, Andhra Pradesh, India
Vibhu Prakash, Bombay Natural History Society, Mumbai, Maharashtra, India
Victor Gapud, University of the Philippines Los Banos, Laguna, Philippines
English Editors
Mrs. Mira Bhojwani, Pune, India
Dr. Fred Pluthero, Toronto, Canada
Mr. P. Ilangovan, Chennai, India
Print copies of the Journal are available at cost. Write
to the Managing Editor, JoTT, c/o Wildlife Information
Liaison Development, 96, Kumudham Nagar, Vilankurichi
Road, Coimbatore, Tamil Nadu 641035, India
threatenedtaxa gmail.com
OPEN ACCESS
All articles published in the Journal of Threatened Taxa are registered under Creative Commons Attribution 4.0 International License unless otherwise mentioned.
JoTT allows unrestricted use of articles in any medium, reproduction and distribution
by providing adequate credit to the authors and the source of publication.
ISSN: 0974-7907 (Online), 0974-7893 (Print)
July 2014 | Vol. 6 | No. 8 | Pages: 6053–6152
ate of P blication
ly
nline Print
DOI: 10.11609/JoTT.26jul14.6053-6152
omm nications
e introd ction of lobally threatened Arabian a elles
Gazella arabica (Pallas, 1766) (Mammalia: Bovidae) in fenced
protected area in central Saudi Arabia
-- M. Zafar-ul Islam, Moayyad Sher Shah & Ahmed Boug,
Pp. 6053–6060
A checklist of mammals of Nepal
-- Sanjan Thapa, Pp. 6061–6072
Eutropiichthys cetosus, a ne ri erine ca ish eleostei
Schilbeidae) from northeastern India
-- Heok Hee Ng, Lalramliana, Samuel Lalronunga &
Lalnuntluanga, Pp. 6073–6081
er ies of ndarban ios here eser e est en al
eastern India: a preliminary survey of their taxonomic
di ersity ecolo y and their conser ation
-- Soumyajit Chowdhury, Pp. 6082–6092
hort omm nication
orthernmost distrib tion of e tree s ecies to the estern
Ghats from the sacred groves of Pune District, Maharashtra,
India
-- Aboli Kulkarni, Mandar N. Datar, Umesh Awasarkar &
Anuradha Upadhye, Pp. 6093–6100
Notes
e additions to the ora of
-- D.S. Rawat, Pp. 6101–6107
ara hand ndia
nterestin lant records from isa ha atnam istrict Andhra
Pradesh, India
-- K. Ravikumar, N. Balachandran, S. Noorunnisa Begum,
P. Patchaimal, Manoranjan Bhanja & K. Lohitasyudu,
Pp. 6108–6121
rther ne additions to the lichen mycota of Andhra
Pradesh, India
-- Satish Mohabe, A. Madhusudhana Reddy, B. Anjali Devi,
Sanjeeva Nayaka & P. Chandramati Shankar, Pp. 6122–6126
A preliminary checklist of odonates in Kerala Agricultural
University (KAU) campus, Thrissur District, Kerala, southern
India
-- C.K. Adarsh, K.S. Aneesh & P.O. Nameer, Pp. 6127–6137
Why the Red Giant Gliding Squirrel Petaurista petaurista
is o en mista en for the amda ha lidin
irrel
Biswamoyopterus biswasi Mammalia odentia ci ridae
in amda ha ational Par Ar nachal Pradesh ndia
-- C. Murali Krishna & Awadhesh Kumar, Pp. 6138–6141
o rolo ical st dy of astrointestinal arasites of ca ti e
animals at an
r ecreational arden and oo in
Bangladesh
-- Most. Monjila Khatun, Nurjahan Begum, Md. Abdullah
Al Mamun, Md. Motahar Hussain Mondal & Md. Shakif-UlAzam, Pp. 6142–6147
A com arati e haematolo ical analysis of Asian le hants
Elephas maximus Linnaeus, 1758 (Mammalia: Proboscidea:
le hantidae mana ed nder di erent ca ti e conditions
in Sri Lanka
-- Ruvinda Kasun de Mel, Devaka Keerthi Weerakoon,
Wanigasekara Daya Ratnasooriya & Ashoka Dangolla,
Pp. 6148–6150
Book Review
e ie of the boo
er ies and Moths of Pa e i er
Reserve’ - Sanjay Sondhi and Krushnamegh Kunte
-- Reviewed by Ullasa Kodandaramaiah, Pp. 6151–6152
Threatened Taxa