Infrarenal abdominal aortic aneurysms associated with proximal
Transcription
Infrarenal abdominal aortic aneurysms associated with proximal
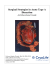
Grand Rounds Vol 3 pages 19–22 Speciality: Vascular Disease Article Type: Case report DOI: 10.1102/1470-5206.2003.0008 c 2003 e-MED Ltd GR Infrarenal abdominal aortic aneurysms associated with proximal dissection A. C. Qureshi, H. S. Flora, M. Matson and R. J. Ham Department of Vascular Surgery, The Royal Hospitals NHS Trust, The Royal London Hospital, London E1 3UU, UK Corresponding address: R. J. Ham, Department of Vascular Surgery, The Royal Hospitals NHS Trust, The Royal London Hospital, London E1 3UU, UK E-mail: rham260823@aol.com Date accepted for publication 18 September 2003 Abstract A case is described presenting with aortic dissection who was found to have a significant infrarenal aortic aneurysm. Various treatment options were considered for both conditions including open operation, conservative management and stent repair. The paieint was managed conservatively for six months whilst the dissection ‘matured’ when the infrarenal aneurysm was repaired by open operation preserving both the true and false proximal lumens to avoid renal or visceral ischaemia. Keywords Aortic dissection; infrarenal aortic aneurysm; treatment. Introduction Laennec first described a ‘dissecting aneurysm’ in 1819 [1] . Surgical management was initially developed by DeBakey et al. in 1955 [2] and within 10 years the role of medical management in the acute setting, by controlling blood pressure and the rate of ventricular contractions, was being recognised [3] . Aortic dissection is characterised by a column of blood separating the inner part of the aortic wall from the outer part of the media and adventitia. An ‘intimal flap’ develops, separating the resulting false lumen from the true lumen [4] . Post dissection, aneurysmal dilatation of the aorta may occur which carries a 20% risk of rupture [2, 5] . The co-existence of both acute dissection of the aorta with an associated infrarenal aortic aneurysm is an uncommon finding at post-mortem or in clinical reviews of aortic pathology [6, 7] and raises important questions concerning management. In the last decade the evolution of surgical techniques has led to the development of endovascular stent grafting for the treatment of aortic aneurysms and these techniques have recently been extended to aortic dissection [8] . However, there are anatomical limitations to these techniques and we present a case which was managed by open repair. Case report A 60-year-old Caucasian gentleman presented with sudden onset of severe intrascapular pain. He was a heavy smoker with known hypertension and hypercholesterolaemia. On clinical examination he was sweaty but without evidence of any haemodynamic compromise. A painless expansile This paper is available online at http://www.grandrounds-e-med.com. In the event of a change in the URL address, please use the DOI provided to locate the paper. 20 A. C. Qureshi et al. Fig. 1. A contrast enhanced CT scan showing the dissection at the origin of the coeliac axis. The true lumen is at the front. Fig. 2. The same scan showing two distinct lumina in the aneurysmal infrarenal aorta. pulsatile abdominal mass was noted. There was no pulse deficit but a chest X-ray showed a widened mediastinum. Computerised tomography (CT) demonstrated a dissection, originating from the aortic arch in the region of the left subclavian artery with extension distally to involve the descending aorta. In addition there was a 6 cm diameter fusiform infrarenal aortic aneurysm involving the common iliac arteries bilaterally (Figs 1 and 2). The visceral and renal arteries arose from both the true and false lumina of the aorta without evidence of visceral or renal ischaemia. The coeliac axis, superior mesenteric artery and right renal artery arose from the true lumen and the left renal artery from the false lumen. Initial management was conservative with a glycerol trinitrate (GTN) infusion, β-blockers and risk factor optimisation. Both the type of surgery and the possibility of the use of stent grafts to both the dissection and the infrarenal aneurysm were carefully considered. The patient remained stable on medical treatment after his presentation. The anatomical complexities of treating the infrarenal aneurysm by stenting together with concern over the friable nature of the aortic wall following dissection suggested that a period of expectant treatment allowing the inflammatory reaction to the dissection to settle was prudent. A period of 6 months was allowed for this whilst the dissection matured and following further assessment of the whole situation it was decided that the infrarenal aneurysm Aortic aneuysm and dissection 21 should be repaired with the availability of cardiothoracic expertise should the dissection extend during surgery and cross-clamping of the suprarenal aorta in particular was needed. The infrarenal aorta was approached via a midline laparotomy incision. Infrarenal control of the aorta was achieved by clamping without incident. The aneurysm was opened, the associated thrombus was removed and a bifurcated graft (Hemashield Gold Knitted Microvel Double Velour Graft) was sutured into both the true and false lumens proximally and to the common iliac arteries distally. The patient made an uncomplicated recovery and was discharged home on the seventh postoperative day. There has been follow-up of over 1 year, with no evidence of proximal dilation of the dissection on the surveillance CT scan at 1 year. Discussion Following aortic dissection, aneurysmal disease of the aorta may lead to an alarming 20% risk of rupture [9] which is mitigated by surgical repair. Current clinical trials of endovascular repair of infrarenal aneurysms have so far been favourable, particularly in selected patients with optimal aortic anatomy [10] . Endovascular techniques have been tested on this variant aortic wall pathology, with potentially lower associated morbidity and mortality. However, this is not an entirely versatile technology in which case open repair has to be considered, as in this situation. The strategy for managing such patients is based upon defining the position of both the exit and re-entry sites of the false lumen. Ideally the former must be excluded as in the open surgical management of a type A dissection. The fact that the dissection was so close to the left subclavian artery meant there was not enough room to land a stent or stent graft without occluding the origin of this vessel. In addition a stent graft system with visceral branches would be necessary; such a device is under development but is not yet commercially available. The strategy employed by Marin et al. [5] has been to force together both the true and false lumen channels at the level of the AAA neck by the deployment of a stent, where the false lumen enters the aneurysm sac. However, where the false lumen re-enters proximal to the aneurysm neck no intervention is undertaken. Marin has not seen any stent graft migration in his series during a mean follow-up of 20 months [5] , implying adequacy of fixation thus far. There are concerns over the stent graft–aortic wall interface adjacent to or within the true or false lumen. Therefore, angiography and/or intravascular ultrasound may be also required for precise positioning of the device, which is not necessary during open repair. In addition, little is known about the long-term healing process of the dissecting aorta once the stent system has been deployed, due to the absence of any histological results in the literature. Clamping of the infrarenal aorta during conventional open repair in these patients [2] may result in expansion, extension or possibly rupture of the dissecting aorta, but this is confined to the peri-operative and immediate post-operative periods. Such scenarios are also possible following endoluminal repair and are an ongoing risk due to an analogous change in the force equilibrium within the dissection caused by alteration of the dissection re-entry site by the stent. Advances in endovascular technologies are exciting and provide increased versatility in treating anatomical variations of aortic wall pathology, but there clearly remain constraints for which open surgery may provide successful solutions in selected patients. Learning point This case demonstrates not only the choice of interventions now available but also the crucial importance of timing in a demanding clinical situation. References 1. Stehbens WE. History of aneurysms. Med History 1958; 2: 274–80. 2. DeBakey ME, McCollum CH, Crawford ES et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred and twenty seven patients surgically. Surgery 1982; 92: 1118–34. 3. Wheat MW, Palmer RF, Bartley TD et al. Treatment of dissecting aneurysms of the aorta without surgery. J Thorac Cardiovasc Surg 1965; 50: 364–73. 4. Flachskampf FA, Daniel WG. Aortic dissection. Cardiol Clin 2000; 18(4): 807–17. 22 A. C. Qureshi et al. 5. Marin ML, Lyon RT, Hollier LH, Kaplan DB. Experience with endovascular grafts in the treatment of infrarenal aortic aneurysms associated with proximal aortic dissection. Am J Surg 1999; 177: 102–6. 6. Miller DC, Mitchell RS, Oyer PE et al. Independent determinants of operative mortality for patients with aortic dissection. Circulation 1984; 70: 1153–64. 7. Yusef SW, Baker DM, Chuter TAM, Whitaker SC. Transfemoral endoluminal repair of abdominal aortic aneurysms with bifurcated graft. Lancet 1995; 344: 350–1. 8. Miyari T, Ninomiya M, Endoh M et al. Conventional repair and operative stent-grafting for acute and chronic aortic dissection. Ann Thorac Surg 2002; 73: 1621–3. 9. Cambira RP, Brewster DC, Gatler J et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg 1998; 7: 199–209. 10. Blum U, Voshage G, Lammer J et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med 1997; 336: 13–20.