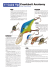vertebrate pesticides - Internet Center for Wildlife Damage
Transcription
vertebrate pesticides - Internet Center for Wildlife Damage
VERTEBRATE PESTICIDES G-1 Registered Vertebrate Pesticides William W. Jacobs G-23 Description of Active Ingredients Robert M. Timm G-24 G-25 G-26 G-30 G-32 G-33 G-34 G-35 G-36 G-37 G-38 G-39 G-40 G-41 G-42 G-43 G-44 G-46 G-47 G-48 G-49 G-52 G-54 G-56 G-57 G-60 Acrolein Aluminum Phosphide Anticoagulants Avitrol® Bone Tar Oil Bromethalin Capsaicin Alpha-Chloralose Chloropicrin Cholecalciferol Denatonium Saccharide Egg Solids, Putrescent Fatty Acids (various compounds) Fenthion Gas Cartridges Methyl Anthranilate Methyl Bromide Naphthalene Red Squill Sodium Cyanide Sodium Fluoroacetate Starlicide® Strychnine Thiram Zinc Phosphide Ziram G-63 Poison Control Centers Blain (Jess) Benson G-67 Sample Labels of Representative Products Scott E. Hygnstrom G-69 Avitrol® G-70 G-71 G-72 G-73 G-74 G-78 G-79 G-80 G-82 G-84 G-85 G-87 G-89 G-90 G-91 ReJeX-iTTM AG-36 ReJeX-iTTM TP-40 ReJeX-iTTM AP-50 Tanglefoot® Bird Repellent Alpha-Chloralose Rid-A-Bird® Perch 1100 Solution PurinaTM StarlicideTM Complete Compound DRC-1339 Concentrate - Feedlots Compound DRC-1339 98% Concentrate - Pigeons 1339 Gull Toxicant 98% Concentrate Compound DRC-1339 98% Concentrate - Livestock Depredations Hinder® Deer and Rabbit Repellent Millere® Hot Sauce® Animal Repellent Ro-pel® Animal, Rodent and Bird Repellent Ro-pel® Garbage ProtectorTM (continued) G-92 G-93 G-94 G-95 G-96 G-98 G-99 G-100 G-101 G-102 G-103 G-104 G-105 G-106 G-107 G-108 G-109 G-110 G-111 G-112 G-113 G-114 G-115 G-116 G-117 G-118 G-119 G-120 G-121 G-122 G-123 G-124 G-125 G-126 G-127 G-128 G-129 G-130 G-132 G-134 G-135 G-136 G-137 G-138 G-140 G-141 G-142 G-145 G-147 G-148 G-149 Bonide® Dogzix Dog and Cat Repellent SudburyTM Chaperone® Squirrel and Bat Repellent Eaton’s® 4 the SquirrelTM Repellent Deer-Away® Big Game Repellent Gustafson Thiram 42-S NOTT Chew-Not Animal Repellent F & B® Rabbit & Dog Chaser Earl May® Rabbit Scat Talon®-G Rodenticide Bait Pack (Pellets) Contrac® Rodenticide Maki® Mini-Block PurinaTM Mouse-A-RestTM Pellets Eaton’s® AC90TM Rodenticide Rozol® Rat and Mouse Killer Rozol® Pocket Gopher Bait Ditrac® Tracking Powder Ditrac® Rat & Mouse Bait Eaton’s® All-Weather Bait Blocks® Liqua-Tox II® Ramik® Green Rodere Paraffinized Rat Bait Eaton’s® AnswerTM for Pocket Gophers Eaton’s® A-C 50TM Rodenticide Final® Rat & Mouse Bait PurinaTM Rat Control Pellets RodexTM BloxTM-1 PurinaTM Assault Rat Place Pack Quintox® Rat and Mouse Bait M-44 Cyanide Capsules Sodium Fluoroacetate (Compound 1080) Livestock Protection Collar 0.5% Strychnine S.R.O. Pocket Gopher Bait Petersens Pocket Gopher Killer I Wilco Gopher Getter AG Bait Bonide® Moletox II Bonide® Orchard Mouse Bait Ridall-ZincTM Tracking Powder Roban II Ag Zinc Phosphide on Wheat for Mouse Control Zinc Phosphide Concentrate for Muskrat and Nutria Control Zinc Phosphide Prairie Dog Bait ZP® Rodent Bait AG ZP® Rodent Bait Place Pack ZP® Tracking Powder Phostoxin® Chlor-o-pic® Chloropicrin Brom-o-gas® The Giant Destroyer® Gas Cartridge for Woodchucks, Ground Squirrels, Prairie Dogs and Pocket Gophers Gas Cartridge for Coyotes Snake-A-Way® Snake Repellent G-151 Index, Chemical and Trade Names Scott E. Hygnstrom G-155 Index, Target Species Scott E. Hygnstrom William W. Jacobs PESTICIDES FEDERALLY REGISTERED FOR CONTROL OF TERRESTRIAL VERTEBRATE PESTS Registration Division Office of Pesticide Programs US Environmental Protection Agency Washington, DC 20460 This report lists the pesticide formulations federally registered for use in controlling vertebrate organisms discussed in this manual. The tables which follow indicate the chemicals registered, the types of formulations available, the number of registered products (as of winter 1993) for each type of formulation, and significant special factors which must be considered prior to using a particular type of product. Species which represent closely related taxonomic groups or similar classes of pests may be listed in the same table. For example the Norway rat, the roof rat, and the house mouse are placed in one table because they are closely related and many labels bear claims for control of all three species. Similarly, products bearing claims for control of ground squirrels are listed in a single table even though some labels claim control of only one or two types. Although these factors have resulted in some adjustments, the order in which registered formulations are listed generally follows the table of contents of this manual (Table 1). Tables 2 through 23 below do not include products registered, under Section 24(c) of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), for use in individual states because of special local needs. The most common Special Local Needs registrations for controlling vertebrate pests are chlorophacinone and diphacinone baits used in post-harvest applications to control voles in orchards or to control ground squirrels. State agencies responsible for regulating pesticides and extension specialists can provide information on the products available only in their states. California residents may consult their county agricultural commissioners. The tables also do not list products which have been or are currently available under the emergency exemption provisions (Section 18) of FIFRA. Extension specialists can provide information on products which are temporarily available for emergency situations. Federal registration certifies that it is legal to market and use the product in question in the United States, subject to the conditions and restrictions stipulated on the approved label. Federal registration does not guarantee that a particular chemical or product will be available throughout the United States or even that the product will actually be marketed. If a particular product cannot be found in a local outlet, the manufacturer should be contacted. If the name of the manufacturer is not known, it can be obtained from your local extension specialist or from Product Management Team 14, Registration Division (H7505C), Office of Pesticide Programs, US Environmental Protection Agency, Washington, DC 20460. Federal registration does not guarantee that a particular pesticide will be effective in controlling a specific pest in every possible situation. This handbook offers guidance for selecting the proper course of action for resolving a particular problem. The product label provides directions for using the product to your best advantage in an environmentally safe manner. Users should read labels carefully before proceeding, and should consult with PREVENTION AND CONTROL OF WILDLIFE DAMAGE — 1994 Cooperative Extension Division Institute of Agriculture and Natural Resources University of Nebraska - Lincoln United States Department of Agriculture Animal and Plant Health Inspection Service Animal Damage Control Great Plains Agricultural Council Wildlife Committee G-1 extension agents if any questions remain after the appropriate section of this manual and the product label have been read. In the tables which follow, most products are identified as “baits.” “Baits” are formulations which are designed to be consumed by the target pests. Baits may be “dry” formulations such as whole grains or pelleted grain mixtures treated with a toxicant, or liquid formulations to be presented for target organisms to drink. “Weatherresistant” baits are dry baits treated with a material such as paraffin which is intended to make the baits resistant to the effects of excessive moisture and/or heat. “Technical” products consist of the active ingredient in pure or relatively pure form. Technicals are usually sold for manufacturing purposes only, but there are a few (see Tables 2 through 23) which can legally be mixed into baits or other types of formulations and applied as pest control agents. Tables 2 through 23 list the available technical vertebrate pesticide formulations only once. Technicals are listed with products for control of commensal rodents (Table 4), unless the chemical in question is not registered for control of Norway rats, roof rats, or house mice. Other technicals are also listed once — under the heading for the type of pests which comprise the principal vertebrate target species grouping for the chemicals in question. Technicals bearing use claims are listed in tables for each pest type claimed. Products identified as “concentrates” are formulated with active ingredient strengths lower than those found in technicals but higher than the concentrations which are used in pest control applications. Concentrates are of two types. One type is sold for manufacturing or formulating purposes only. The labels for these products bear no use directions. It is unlawful to use such products directly in pest control operations. Such concentrates can be formulated into products which can be registered and sold as pesticides. The second type of concentrate bears label G-2 directions for mixing and applying the product. In vertebrate pest control, this type of concentrate enables the experienced worker to mix baits suited to the control of a particular infestation. “Restricted Use Pesticides” are products which may be used only by persons who have been trained and certified to use them. Nearly all pesticide-user certification programs are run by state governments. A pesticide may be classified as “restricted” because the US Environmental Protection Agency has determined that the product is highly toxic and must be handled especially carefully or that special training is necessary to ensure that the material is used effectively or in a safe manner. Other significant terms in the tables are consistent with their use elsewhere in this manual. Within a species grouping, products are classed under use category headings such as “Toxicant,” “Fumigant,” and “Repellent.” In some cases, prod- ucts of different formulations or even different chemical compositions have been grouped together as one table entry. Such grouping has occurred for gas cartridge fumigants with more than one product for a species or type and for “polybutene” bird repellents. The abbreviation “Conc.” in Tables 2 through 23 stands for “concentration.” In Tables 2 through 23, the column titled “Use Considerations and Restrictions” highlights factors that must be considered in determining whether a product is appropriate for use in a given situation. These limitations are fully explained on product labels. Do not use any pesticide until you have thoroughly read and understood its label. Acknowledgments The author wishes to thank William Erickson and Barbara Madden for assistance in updating the lists of vertebrate pesticides. Table 1. Tables where federally registered pesticide formulations for controlling terrestrial vertebrates can be found. Animal Type(s) Beavers Chipmunks Gophers, Pocket Mice, Deer Mice, Harvest Mice, House Mice, Pocket Mice, White-footed Mountain Beavers Muskrats Nutria Porcupines Prairie Dogs Rats, Cotton Rats, Kangaroo Rats, Norway Rats, Polynesian Rats, Roof Rats, Water Woodrats Squirrels, Ground Squirrels, Tree Voles Woodchucks Badgers Bears Bobcats House Cats Table Number 2 13 3 6 None 4 6 6 None 7 7 8 9 10 10 4 5 4 10 10 11 14 6 12 None None None 15 Animal Type(s) Coyotes Dogs Foxes Mink Mountain Lions Raccoons River Otters Skunks Weasels Wolves Armadillos Bats Deer Elk Moles Opossums Pronghorn Antelope Pigs, Wild Rabbits Hares Shrews Birds Alligators Crayfish Frogs & Toads Salamanders Snakes Turtles Table Number 16 15, 16 16 None None None None 17 None None None 18 19 19 20 None None None 21 21 None 22 None None None None 23 None Table 2. Pesticide federally registered to repel beavers. DENATONIUM SACCHARIDE Conc. Product Form 0.065%* Liquid No. of Products Use Considerations and Restrictions 1 Taste repellent used to protect trees, poles, fences, siding, outdoor furniture, shrubs, ornamentals, grass, flowers, and garbage * Also contains 0.035% thymol Table 3. Pesticides federally registered to control pocket gophers. TOXICANTS CHLOROPHACINONE (RoZol) Conc. Product Form Species 0.005% Dry bait Geomys spp. Thomomys spp. No. of Products 1 Use Considerations and Restrictions Subterranean applications DIPHACINONE (Eaton’s Answer) Conc. Product Form Species 0.005% Dry bait (block) Geomys bursarius Thomomys bottae T. talpoides T. mazama T. bulbivorous T. monticola T. townsendii No. of Products 1 Use Considerations and Restrictions Subterranean applications STRYCHNINE ALKALOID Conc. Product Form Species No. of Products 0.25%-0.5% Dry baits Geomys spp. Thomomys spp. 0.35% Dry bait Unspecified 1 Manual subterranean applications only 0.5% Dry baits Plains Desert Yellow-faced Northern Southern Mazama Mountain Townsend’s Botta Camas 2 Manual subterranean applications only 0.31%-0.5% Dry baits Geomys spp. Thomomys spp. 3 Restricted Use Pesticides Subterranean applications 0.5% Dry baits Thomomys spp. 2 Restricted Use Pesticides Subterranean applications 0.5% Dry baits Plains Yellow-faced Northern Southern Mazama Mountain Townsend’s Botta Camas 2 Restricted Use Pesticides Subterranean applications 0.35% Dry bait Unspecified 1 Restricted Use Pesticide Subterranean applications 98.4%-100% Technical 1 Manufacturing use 5 Use Considerations and Restrictions* Manual subterranean applications only * Strychnine alkaloid pocket gopher baits are classified as “Restricted Use Pesticides” if their labels permit applications by machine-drawn burrow builders. All strychnine alkaloid pocket gopher baits must be applied underground. G-3 Table 3. (Continued) ZINC PHOSPHIDE Conc. Product Form Species No. of Products Use Considerations and Restrictions 2% Dry baits (bulk) Geomys spp. Thomomys spp. 6 Subterranean use 2% Dry bait (placepack) Geomys spp. Thomomys spp. 1 Subterranean use 2% Dry baits Geomys bursarius Geomys pinetis Pappogeomys castanops Thomomys spp. 2 Restricted Use Pesticides* * Products are classified as “Restricted Use Pesticides” due to directions on labels for using burrow builders and claims for control of species other than pocket gophers for which zinc phosphide baits are “restricted.” FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species 55%-60% Tablets, Pellets, or Bags Unspecified No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows GAS CARTRIDGES (many active ingredients) Conc. Product Form Species Various Ignitable cartridges Unspecified G-4 No. of Products 4 Use Considerations and Restrictions For treating burrows Table 4. Pesticides federally registered to control commensal rodents: Norway rats, roof rats, house mice. CHRONIC TOXICANTS BRODIFACOUM (Talon) - 3-[3-(4'-bromo[l,l’-biphenyl]4-yl)-l,2,3,4-tetra-hydro-l-naphthalenyl]4-hydroxy-2H-l-benzopyran-2-one Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse Use Considerations and Restrictions 0.005% Dry baits (bulk) 7 X X X In and around buildings 0.005% Dry baits (in placepacks) 14 X X X In and around buildings 0.005% Dry baits (in bait trays) 2 X X X In and around buildings 0.005% Dry baits (in nonprotective bait dispensers) 4 X In and around buildings 0.005% Dry bait (in tamper-resistant bait stations) 1 X In and around buildings 0.005% Weather-resistant baits 2 X In and around buildings, sewers 0.25% Concentrate 1 Formulating use 90% Technical 1 Manufacturing use X X BROMADIOLONE (Maki, Contrac) - 3-[3-(4'-bromo[l,l’-biphenyl]-4-yl)-3-hydroxy-l-phenylpropyl]-4-hydroxy-2H-l-benzopyran-2-one Conc. Product Form No. of Products Norway rat X Species Claimed Roof House rat mouse 0.005% Dry baits (bulk) 7 0.005% Dry bait (bulk) 1 0.005% Dry baits (in placepacks) 5 0.005% Dry bait (in nonprotective bait dispensers) 1 0.005% Weather-resistant baits 3 1.0% Concentrates 2 Formulating use 93.5%-96.5% Technicals 2 Manufacturing use X X X Use Considerations and Restrictions X X X In and around urban buildings; indoor use only in other areas X In and around urban buildings. Indoor use only in other areas. X In and around urban buildings. Indoor use only in other areas. X In and around urban buildings. Indoor use only in other areas. X In and around urban buildings. Indoor use only in other areas. CALCIUM SALT OF PMP - 2-isovaleryl-1,3-indandione, calcium salt Conc. 2.18% Product Form Tracking powder No. of Products 1 Norway rat X Species Claimed Roof House rat mouse X Use Considerations and Restrictions X CHLOROPHACINONE (RoZol) - 2-[(p-chlorophenyl)phenylacetyl]-1,3-indandione Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse 0.005% Dry baits (bulk) 5 X X X 0.005% Dry baits 2 X X X 0.005% Weather-resistant bait 1 X X X Use Considerations and Restrictions 0.2% Tracking powders 2 X X X Restricted Use Pesticides 0.28% Mineral oil concentrate 1 X X X For mixing dry baits 2% Concentrate 1 Formulating use 96.03% Technical 1 Manufacturing use G-5 Table 4. (Continued) CHOLECALCIFEROL* (Quintox) 9,10-seocholesta-5,7,10(19)-trein-3-betaol Conc. Product Form No. of Products 0.075% Dry bait (bulk) 1 0.075% Dry bait (bulk) 1 0.075% Dry bait (placepacks) 1 0.075% Dry bait (placepacks) 1 Norway rat X X Species Claimed Roof House rat mouse X X Use Considerations and Restrictions X In and around buildings X In and around buildings X In and around buildings X In and around buildings * Cholecalciferol is the only chronic poison listed in this table that is not an anticoagulant. DIPHACINONE (Ditrac, Ramik) - 2-(diphenylacetyl)-l,3-indandione Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse Use Considerations and Restrictions 0.005% Dry baits (bulk) 13 X X X 0.005% Dry baits (in placepacks) 6 X X X 0.005% Dry bait (in non-protective bait stations) 1 0.005% Weather-resistant dry baits 13 X X 0.005% Weather-resistant dry baits 11 X X 0.1% Concentrates 3 X X X Mixing dry baits 0.1% Concentrates 2 X X X Manufacturing use 0.2% Tracking powders 2 X X X Restricted Use Pesticides 2.0% Concentrate 1 Formulating use 98.0%-98.6% Technicals 3 Manufacturing use X X Can be used in damp/moist areas Can be used in damp/moist areas SODIUM SALT OF DIPHACINONE 0.1% Concentrate 1 X X X Mixing dry baits 0.106% Concentrates 2 X X X Mixing liquid baits PIVALYL (Pival, Pindone) - 2-pivalyl-l,3-indandione Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse 0.025% Dry baits (bulk) 6 X X 0.025% Dry baits 2 X X 0.30% Concentrate 1 X X X 0.50% Concentrates 2 X X X 100% Technical 1 Use Considerations and Restrictions X Mixing liquid and dry baits Mixing dry baits Manufacturing use SODIUM SALT OF PIVALYL 0.14% Concentrates 2 X X X Mixing liquid baits 1.5% Concentrate 1 X X X Mixing liquid baits 100% Technical 1 Formulation use PIVALYL AND SODIUM SALT OF PIVALYL MIXTURE 0.11% Pivalyl 0.15% Sodium salt G-6 Dry bait 1 X X X Table 4. (Continued) WARFARIN - 3-(α α-acetonylbenzyl)-4-hydroxycoumarin Conc. Product Form No. of Products Norway rat 0.025% Dry baits (bulk) 23 X 0.025% Dry bait (bulk) 1 0.025% Dry baits (in placepacks) 4 X 0.025% Dry baits (in bait trays) 2 0.025% Dry bait (in nonprotective bait stations) 0.025% Species Claimed Roof House rat mouse X Use Considerations and Restrictions X In and around buildings X In and around buildings X X In and around buildings X X X In and around buildings 1 X X X Weather resistant 1 X X X In and around buildings and in sewers 0.054% Dry bait (in nonprotective bait stations) 1 X In and around buildings 0.3% Concentrate 1 X X X Mixing dry baits 0.5% Concentrates 2 X X X Mixing dry baits 0.5% Concentrates 4 Formulating use 5% Concentrate 1 Manufacturing use 50% Technical (encapsulated) 1 Manufacturing use 99.9%-100% Technicals 5 Manufacturing use SODIUM SALT OF WARFARIN 0.54% Concentrate 1 X X X Mixing liquid baits ACUTE TOXICANTS BROMETHALIN (Assault, Vengeance) - N-methyl-2,4-dinitro-N-(2,4,6-tribromophenyl)-6-(trifluoromethyl) benzenamine Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse Use Considerations and Restrictions 0.005% Dry bait (bulk) 1 X X In and around buildings, alleys 0.01% Dry baits (bulk) 2 X X X In and around buildings, alleys 0.01% Dry bait (bulk) 1 0.01% Dry baits (in placepacks) 3 X In and around buildings, alleys X X X In and around buildings, alleys 0.01% Dry bait (in placepacks) 1 X X 0.01% Dry bait (in placepacks) 1 X In and around buildings, alleys 0.01% Dry bait (in nonprotective bait stations) 1 X Indoor use only 0.01% Weather-resistant bait 1 1.5% Concentrate 1 Formulating Use 2% Concentrate 1 Formulating Use X X In and around buildings, alleys In and around buildings, alleys G-7 Table 4. (Continued) RED SQUILL Conc. 10% Product Form No. of Products Bait in caulking tube 1 Norway rat X Species Claimed Roof House rat mouse Use Considerations and Restrictions X ZINC PHOSPHIDE Conc. Product Form No. of Products Norway rat Species Claimed Roof House rat mouse Use Considerations and Restrictions 1.0% Dry bait 1 X X X Restricted Use Pesticide - structural 1.88%-2% Dry baits 2 X X X Restricted Use Pesticides - sugarcane 1.88%-2% Dry baits 5 X X X Restricted Use Pesticides - sugarcane and other uses 1.88%-2% Dry baits 3 X X X Structural uses only 2% Dry bait 1 X X Structural uses only 2% Dry bait 1 X X Restricted Use Pesticide - structural 10% Tracking powders 2 X Restricted Use Pesticides - indoor use 63.2% Concentrate 1 X X 74.7% Concentrate 1 X X X Restricted Use Pesticide for making baits 80%-94% Technicals 2 X X X Restricted Use Pesticides for making baits 80%-94% Technicals 5 X Restricted Use Pesticide for making baits Manufacturing use FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. 55%-60% Product Form No. of Products Tablets, pellets, or bags Norway rat Species Claimed Roof House rat mouse Use Considerations and Restrictions 18 X X X Restricted Use Pesticides for treating burrows 2 X X X Restricted Use Pesticides for treating structures CHLOROPICRIN (Chlor-o-pic) 99% Liquids GAS CARTRIDGES (Many active ingredients) Various Ignitable cartridges w/fuse 2 X For treating burrows 16 X X X Restricted Use Pesticides for treating structures X X X Taste repellent used on trees, poles, fences, shrubs, garbage, and other objects METHYL BROMIDE (Brom-o-gas) 98%-100%* Gases * Some products contain small amounts of chloropicrin. REPELLENT DENATONIUM SACCHARIDE (Ro-pel) 0.065%* Liquid * Also contains 0.035% thymol G-8 1 Table 5. Pesticides federally registered to control polynesian rats.* ACUTE TOXICANT ZINC PHOSPHIDE Conc. Product Form 1.88%-2% Dry baits** No. of Products Use Restrictions and Considerations 5 Restricted Use Pesticides for use in sugarcane fields * In the United States, this species is found only in Hawaii. ** Prebaiting is recommended. Table 6. Pesticides federally registered to control native mice (voles, deer mice, and others) TOXICANTS ZINC PHOSPHIDE Conc. Product Form Species No. of Products Use Considerations and Restrictions 1.0% Dry bait Meadow voles Pine voles 1 Restricted Use Pesticide for post-harvest use in orchards and other sites 1.82% Dry bait Voles (Microtus spp.) 1 Restricted Use Pesticide for post-harvest use in orchards and noncrop areas 1.82% Dry bait Meadow voles Prairie voles Pine voles White-footed mice 1 Restricted Use Pesticide for post-harvest use in orchards and other sites 1.88% Dry bait Meadow voles Pine voles 1 Restricted Use Pesticide for post-harvest use in orchards and other sites 2.0% Dry bait Meadow voles Pine voles 1 Restricted Use Pesticide for post-harvest use in apple orchards 2.0% Dry bait Microtus spp. Peromyscus spp. 1 Restricted Use Pesticide for use in grape vineyards, orchards, and other sites 2.0% Dry baits Meadow voles Pine voles White-footed mice 3 Restricted Use Pesticides for post-harvest use in orchards and other sites 2.0% Dry bait Meadow voles Pine voles 1 Restricted Use Pesticide for post-harvest use in orchards and other sites 2.0% Dry bait Meadow voles Pine voles White-footed mice Pocket mice 1 Restricted Use Pesticide for post-harvest use in orchards and other sites 2.0% Dry baits Meadow voles Prairie voles Pine voles White-footed mice Meadow jumping mice 3 Restricted Use Pesticides for use in grape vineyards, orchards, and other sites 62% Concentrate “Orchard mice” 1 For making baits with “cubed apples” 63.2% Concentrate Meadow voles Pine voles White-footed mice 1 Restricted Use Pesticide for making baits with “cubed apples” for post-harvest use in orchards G-9 Table 6. (Continued) FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species No. of Products 55%60% Tablets, pellets, or bags Voles Conc. Product Form Species 2.5% Liquid Meadow voles Pine voles Conc. Product Form Species 7% Liquids “Meadow mice” 2 Ornamental plants and trees 20% Liquid concentrates “Meadow mice” 3 Nursery stock, ornamentals, fruit trees, and other sites 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows REPELLENTS CAPSAICIN No. of Products 1 Use Considerations and Restrictions Ornamental trees, shrubs, dormant fruit and nut trees, vines, and certain crops THIRAM NOTE: G-10 No. of Products Use Considerations and Restrictions Labels vary in the use of common or scientific names for native mice. This table generally portrays species claims as they appear on the labels. Scientific names are used in this table only if common names for species do not appear on labels. The term “pine vole” refers to Microtus (or Pitymys) pinetorum. “Meadow vole” technically refers only to Microtus pennsylvanicus, but at times has been applied to other Microtus species (except M. pinetorum). Peromyscus species include deer mice and white-footed mice, and related types. Pocket mice are Heteromyid rodents of the genus Perognathus. Jumping mice are of the genus Zapus. “Orchard mice” — a poorly descriptive term — is taken to include voles and Peromyscus species. Table 7. Pesticide federally registered to control muskrats and nutria. TOXICANT ZINC PHOSPHIDE Conc. Product Form Species 63.2% Concentrate Muskrats Nutria No. of Products 1 Use Considerations and Restrictions Restricted Use Pesticide for making baits to be placed on floating rafts NOTE: This is a grouping of convenience due to ecological similarities between muskrats and nutria. It is not a taxonomic grouping. Table 8. Pesticide federally registered to repel porcupines. REPELLENT CAPSAICIN Conc. Product Form 2.5% Liquid concentrate Number of Products Use Considerations and Restrictions 1 To be applied to maple sap collection equipment Table 9. Pesticides federally registered to control prairie dogs. TOXICANTS ZINC PHOSPHIDE Conc. Product Form Species 1.88%-2.0% Dry baits* Black-tailed White-tailed Gunnison No. of Products 7 Use Considerations and Restrictions Restricted Use Pesticides * Prebaiting is recommended. FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species* 55%-60% Tablets, pellets, or bags Black-tailed White-tailed Gunnison No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows * Labels claim “Prairie dogs (except Utah prairie dogs).” GAS CARTRIDGE (many active ingredients) Conc. Product Form Species Various Ignitable cartridge Unspecified No. of Products 1 Use Considerations and Restrictions For treating burrows G-11 Table 10. Pesticides federally registered to control native rats (woodrats, cotton rats, kangaroo rats, and others). TOXICANTS ZINC PHOSPHIDE Conc. Product Form Species No. of Products Use Considerations and Restrictions 2.0% Dry baits Cotton rats Rice rats Florida water rats Merriam’s, Ord’s, & banner-tailed kangaroo rats 3 Restricted Use Pesticides for use in sugarcane (first three claims) and rangeland vegetation and noncrop areas (latter three claims) 2.0% Dry bait Cotton rats 1 Trees and grassy areas (implied) 2.0% Dry bait Cotton rats 1 Sugarcane 2.0% Dry bait Cotton rats Rice rats 1 Sugarcane Table 11. Pesticides federally registered to control ground squirrels. TOXICANTS ZINC PHOSPHIDE Conc. Product Form Species No. of Products Use Considerations and Restrictions 1.0% Dry bait* Columbian Richardson’s 13-lined 1 Restricted Use Pesticide 1.88% Dry bait* California Richardson’s 13-lined 1 Restricted Use Pesticide 2.0% Dry bait* California 1 Restricted Use Pesticide 2.0% Dry baits* California Columbian Richardson’s 13-lined 3 Restricted Use Pesticides 2.0% Dry bait* Belding’s California Columbian Golden-mantled Richardson’s 13-lined Townsend’s Uinta Washington 1 Restricted Use Pesticide * Prebaiting is recommended. FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species 55%-60% Tablets, Pellets, or Bags Unspecified No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows GAS CARTRIDGES (many active ingredients) Conc. Product Form Species Various Ignitable cartridges Unspecified G-12 No. of Products 6 Use Considerations and Restrictions For treating burrows Table 12. Pesticides federally registered to control woodchucks and yellowbelly marmots (rockchucks). FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species 55%-60% Tablets, pellets, or bags Woodchucks Yellowbelly marmots No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows GAS CARTRIDGES (many active ingredients) Conc. Product Form Species Various Ignitable cartridges Woodchucks No. of Products 4 Use Considerations and Restrictions For treating burrows NOTE: Consult state and local wildlife laws before using any pesticide to control woodchucks or yellowbelly marmots. Table 13. Pesticides federally registered to control chipmunks. FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species 55%-60% Tablets, pellets, or bags Unspecified Conc. Product Form Species 21% Liquid Unspecified No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows REPELLENT THIRAM No. of Products 1 Use Considerations and Restrictions Shrubs, ornamentals, and dormant fruit trees G-13 Table 14. Products federally registered to control tree squirrels. REPELLENTS CAPSAICIN Conc. Product Form Species 2.5% Liquid concentrate Gray squirrels “Black” squirrels Fox squirrels Red squirrels DENATONIUM SACCHARIDE (Ro-pel) Conc. Product Form Species 0.065%* Liquid Unspecified squirrels No. of Products 1 No. of Products 1 Use Considerations and Restrictions To be applied to maple sap collection equipment Use Considerations and Restrictions Taste repellent used to protect seeds, bulbs, trees, poles, fences, siding, outdoor furniture, shrubs, ornamentals, grass, flowers, and garbage * Also contains 0.035% Thymol NAPHTHALENE Conc. Product Form Species 100% Flakes Unspecified squirrels Conc. Product Form Species 90% Tacky gel Unspecified squirrels No. of Products 1 Use Considerations and Restrictions Attics, wall, and floor voids POLYBUTENES G-14 No. of Products 1 Use Considerations and Restrictions Buildings and “adjacent inanimate structures” Table 15. Federally registered dog and cat repellents. PRODUCTS CLAIMED TO PROVIDE INDOOR OR OUTDOOR AREA OR SPOT REPELLENCY, CESSATION OF URINATION OR DEFECATION, OR PROTECTION OF GARBAGE CONTAINERS ANISE, OIL OF Conc. Product Form Species 1.6% Liquid Dogs and cats Conc. Product Form Species 4.015%* Granular Dogs No. of Products 1 Use Considerations and Restrictions Outdoor use to prevent defecation, urination, and digging (cats only) in lawns, gardens, and flower and shrub beds d-LIMONENE No. of Products 1 Use Considerations and Restrictions Outdoor use to prevent defecation in lawns, flower gardens, driveways, sidewalks, and other areas * Also contains 0.049% dihydro-5-heptyl-2(3H)-furanone and 0.024% dihydro-5-pentyl-2(3H)-furanone METHYL NONYL KETONE* Conc. Product Form Species No. of Products Use Considerations and Restrictions 0.08% Granular Dogs and cats 1 Outdoor use to repel from ornamental plants, trees, shrubs, flowers, and vegetables 1.8% “Water crystals” Dogs and cats 1 Outdoor use to prevent urination and defecation on or near trees, shrubs, and flowers, or in yards 1.9% Sprays Dogs only Cats only Dogs and cats 1 1 31 Indoor use to protect rugs and furniture and/or outdoor use to prevent urination or defecation on or near trees, shrubs, and flowers, or in yards, and (in some cases) to repel dogs and cats from garbage cans and/or trash bags 1.9% Granulars Dogs and cats 2 Outdoor use to protect trees, shrubbery, lawns, and flower beds from urination or defecation, and to repel dogs and cats from garbage cans 47.5%-95% Concentrates or Technicals 3 Manufacturing use * Methyl nonyl ketone products also contain compounds “related” to methyl nonyl ketone at an aggregate concentration of about 5% of the concentration declared for methyl nonyl ketone. NAPHTHALENE Conc. Product Form Species 15%* Granular Dogs No. of Products 1 Use Considerations and Restrictions Outdoor use to repel dogs from lawns, flowers, shrubs, and fences * Also contains 5.0% dried blood, 4.0% thiram, and 0.5% nicotine. TOBACCO DUST Conc. Product Form Species 70%* Dry mixtures Dogs No. of Products 2 Use Considerations and Restrictions Border treatment for protecting trees, shrubs, and vegetable gardens * Also contains 15% dried blood and 15% naphthalene. NO-CHEW PRODUCT BITREX Conc. Product Form Species 0.065%* Spray Dogs No. of Products 1 Use Considerations and Restrictions For use on furniture, rugs, and other “household articles” * Also contains 1.3% thymol, and 1.3% essential oils. G-15 Table 15 (Continued) ATTACK REPELLENTS CAPSAICIN (Oleoresin of Capsicum) Conc. Product Form Species 0.35%-1.0% Sprays Dogs No. of Products 4 Use Considerations and Restrictions Protection from attack DENATONIUM SACCHARIDE (Ro-pel) Conc. Product Form Species No. of Products 0.065%* Liquid Dogs and cats 1 Use Considerations and Restrictions For use on trees, poles, fences, siding, outdoor furniture, similar objects, shrubs, ornamental plants, grass, flowers, and garbage * Also contains 0.035% Thymol Table 16. Pesticides federally registered to control coyotes, foxes, and wild dogs. TOXICANTS SODIUM CYANIDE Conc. Product Form 88.78% Powder packed in capsules 91.06% No. of Products Species Use Considerations and Restrictions* 1 Coyotes Wild dogs Restricted Use Pesticide for use in M-44 ejector device (livestock protection only) Powder packed in capsules 1 Coyotes Gray fox Red fox Wild dogs Restricted Use Pesticide for use in M-44 ejector device (livestock protection only) 91.06% Powder packed in capsules 4 Coyotes Gray fox Red fox Wild dogs Restricted Use Pesticide for use in M-44 ejector device (livestock and endangered species protection) 91.06% Powder packed in capsules 1 Coyotes Gray fox Red fox Wild dogs Restricted Use Pesticide for use in M-44 ejector device (livestock and endangered species protection, and control of vectors of communicable diseases) * Consult specific labels and technical bulletins for information pertaining to where products may be used, who may use them, and detailed restrictions on use. Specific training and state certification is required. SODIUM FLUOROACETATE (Compound 1080) Conc. Product Form No. of Products Species Use Considerations and Restrictions* 1.00%-1.04% Solutions in “Livestock Protection Collar” 6 Coyotes Restricted Use Pesticides with specific training and state certification required (collars are placed on necks of sheep and goats in fenced pastures) 90.0% Technical 1 None May only be used to formulate solutions for federally registered “Livestock Protection Collars” * Consult specific labels and technical bulletins for information pertaining to where products may be used, who may use them, and detailed restrictions on use FUMIGANT GAS CARTRIDGE (many active ingredients) Conc. Product Form Various* Ignitable cartridge * Also contains 35% charcoal. G-16 No. of Products Species Use Considerations and Restrictions 1 Coyotes For use in dens only Table 17. Pesticides federally registered to control skunks. FUMIGANTS GAS CARTRIDGES (many active ingredients) Conc. Product Form Species Various Ignitable cartridges Unspecified skunks No. of Products 2 Use Considerations and Restrictions For treating burrows Table 18. Pesticides federally registered to control bats. REPELLENTS NAPHTHALENE Conc. Product Form Species 100% Flakes Unspecified bats No. of Products 4 Use Considerations and Restrictions For use in attics and wall voids G-17 Table 19. Pesticides federally registered to repel deer and elk. AMMONIUM SOAPS OF HIGHER FATTY ACIDS (Hinder) Conc. Product Form Species No. of Products Use Considerations and Restrictions 15% Liquid concentrate Unspecified deer 1 Fruit trees, vines, field crops, forage crops, grain crops, ornamentals, nursery stock, vegetables, and noncrop areas 15% Liquid concentrate Unspecified deer 1 Flowers, trees, vines, lawns, ornamentals, and vegetables BONE TAR OIL (Magic Circle) Conc. Product Form Species 93.75% Liquid concentrate Unspecified deer No. of Products 1 Use Considerations and Restrictions Grain and forage crops, fruit and shade trees, shrubs, flowers, vegetable gardens, and nursery stock CAPSAICIN (Hot Sauce) Conc. Product Form Species No. of Products 2.5% Liquid concentrate “Deer” 1 Use Considerations and Restrictions Ornamental trees, shrubs, vines, nursery stock, dormant fruit and nut trees, and certain crops DENATONIUM SACCHARIDE (Ro-pel) Conc. Product Form Species 0.065%* Liquid Unspecified deer No. of Products 1 Use Considerations and Restrictions For use on trees, poles, fences, siding, outdoor furniture, similar objects, shrubs, ornamental plants, grass, flowers, and garbage * Also contains 0.035% thymol EGG SOLIDS, PUTRESCENT WHOLE (Deer-Away) Conc. Product Form Species 36% Powder White-tailed deer No. of Products 1 Black-tailed deer Mule deer Roosevelt elk 37% Liquid concentrates White-tailed deer Use Considerations and Restrictions Various ornamentals, almond, fruit, and citrus trees Conifer seedlings 2 Mule deer Black-tailed deer Roosevelt elk Various ornamentals, nonbearing fruit, and citrus trees Conifer seedlings THIRAM Conc. Product Form Species No. of Products 7% Liquid “Deer” 1 Dormant fruit trees, shrubs, and ornamentals 11% Liquid “Deer” 1 Shrubs, fruit trees, ornamentals, and nursery stock 20%-21% Liquids Unspecified 5 Dormant fruit trees, shrubs, and ornamentals 42% Liquids Unspecified 2 Fruit trees, shrubs, ornamentals, and nursery stock G-18 Use Considerations and Restrictions Table 20. Pesticides federally registered to control moles. TOXICANT STRYCHNINE ALKALOID Conc. Product Form Species 0.5% Dry bait Unspecified No. of Products 1 Use Considerations and Restrictions Subterranean applications FUMIGANTS ALUMINUM PHOSPHIDE (Phostoxin) Conc. Product Form Species 55%-60% Tablets, pellets, or bags Unspecified No. of Products 16 Use Considerations and Restrictions Restricted Use Pesticides for treating burrows GAS CARTRIDGES (many active ingredients) Conc. Product Form Species Various Ignitable cartridges Unspecified No. of Products 4 Use Considerations and Restrictions For treating burrows Table 21. Pesticides federally registered to control rabbits. REPELLENTS AMMONIUM SOAPS OF HIGHER FATTY ACIDS (Hinder) Conc. Product Form Species No. of Products 15% Liquids Unspecified rabbits Conc. Product Form Species 2.5% Liquid Unspecified rabbits Conc. Product Form Species 7.0% Liquid Unspecified rabbits 1 Ornamentals 11.0% Liquid Unspecified rabbits 1 Shrubs, fruit trees, ornamentals, and nursery stock 20%-21% Liquids Unspecified rabbits 5 Dormant fruit trees, shrubs, ornamentals, and other sites (claims vary somewhat among products) 42% Liquids Unspecified rabbits 2 Fruit trees, shrubs, ornamentals, and nursery stock Conc. Product Form Species 70%* Dry mixtures Unspecified rabbits 2 Use Considerations and Restrictions Fruit trees, flowers, vegetables, ornamentals, and other sites CAPSAICIN No. of Products 1 Use Considerations and Restrictions Ornamentals, dormant fruit and nut trees, fruit bushes and vines, and certain edible crops THIRAM No. of Products Use Considerations and Restrictions TOBACCO DUST No. of Products 2 Use Considerations and Restrictions Border treatment for protecting trees, shrubs, and vegetable gardens * Also contains 15% dried blood and 15% naphthalene. ZIRAM Conc. Product Form Species 23% Dust (to be applied as is or to be mixed and sprayed) Unspecified rabbits No. of Products 1 Use Considerations and Restrictions Flowers, shrubs, and certain garden fruits and vegetables NOTE: Labels that state “rabbits” are taken to refer to “true rabbits” as well as “hares.” G-19 Table 22. Pesticides federally registered to control birds. TOXICANTS FENTHION (Rid-A-Bird 1100) Conc. Product Form Species 11% Liquid Starlings House sparrows Pigeons No. of Products 1 Use Considerations and Restrictions Restricted to “persons trained in bird control” to be used only in special perches placed where stipulated on label STARLICIDE (Compound DRC-1339) Conc. Product Form Species No. of Products Use Considerations and Restrictions 0.1% Dry bait Starlings Blackbirds 1 For use around livestock and poultry operations under direction of personnel trained in bird damage control 97% Technical Starlings Blackbirds 1 For formulation, by state and federal agencies, into “experimental baits suitable for starling and blackbird control” 98% Technical with use directions Starlings Brewer’s blackbirds Red-winged blackbirds Common grackles Brown-headed cowbirds 1 Restricted Use Pesticide for use by or under direct supervision of US Department of Agriculture employees in cattle, poultry, or hog feedlots 98% Technical with use directions Herring gulls Great black backed gulls Ring-billed gulls 1 Restricted Use Pesticide for use by or under direct supervision of US Department of Agriculture employees in or near nesting colonies of certain types of birds within coastal areas between March 1 and June 30 98% Technical with use directions Pigeons 1 Restricted Use Pesticide for use by or under direct supervision of US Department of Agriculture employees on flat rooftops or within fenced areas from which the public and pets, domestic animals, and most non-avian wildlife can be excluded No. of Products FRIGHTENING AGENTS 4-AMINOPYRIDINE (Avitrol) Conc. Product Form Species 0.03% Dry bait Common grackles Red-winged blackbirds Yellow-headed blackbirds Cowbirds Starlings 1 Restricted Use Pesticide to be used to protect ripening sweet corn and field corn 0.03% Dry bait Common grackles Red-winged blackbirds Rusty blackbirds Yellow-headed blackbirds Cowbirds Starlings 1 Restricted Use Pesticide to be used to protect ripening sunflower fields 0.3% Concentrate 1 Formulation and repackaging 0.5% Dry baits* House sparrows Grackles Cowbirds Red-winged blackbirds Yellow-headed blackbirds Rusty blackbirds Brewer’s blackbirds Starlings 3 Restricted Use Pesticides for use in, on, or in the area of structures, nesting, feeding, and roosting sites 0.5% Dry bait* House sparrows Grackles Cowbirds Red-winged blackbirds Yellow-headed blackbirds Rusty blackbirds Brewer’s blackbirds Starlings Pigeons 1 Restricted Use Pesticide for use in, on, or in the area of structures, nesting, feeding, loafing, and roosting sites G-20 Use Considerations and Restrictions Table 22. (Continued) 4-ANIMOPYRIDINE (Avitrol) (Continued) 0.5% Dry bait* Pigeons 1 Restricted Use Pesticide for use in, on, or in the area of structures, feeding, nesting, loafing, and roosting sites 0.8% Dry bait* Starlings 1 Restricted Use Pesticides for use in, on, or in the area of feedlots, structures, nesting, feeding, and roosting sites 1.0% Dry bait* House sparrows Grackles Cowbirds Red-winged blackbirds Yellow-headed blackbirds Rusty blackbirds Brewer’s blackbirds Starlings 1 Restricted Use Pesticides for use in, on, or in the area of feedlots, structures, nesting, feeding, and roosting sites 1.0% Dry bait* Crows 1 Restricted Use Pesticide for use in, on, or in the area of structures, feeding, nesting, and roosting sites 25% Concentrate powder to be mixed* Herring gulls 1 Restricted Use Pesticide to be applied at, in, or near sanitary landfills 50% Concentrate powder to be mixed* Starlings 1 Restricted Use Pesticide to be used in or near feedlots * Prebaiting is recommended REPELLENTS CAPSICUM (Ground red peppers) Conc. Product Form Species 12%* Granulars Horned larks House finches Unspecified sparrows Starlings No. of Products 2 Use Considerations and Restrictions For use on certain fruit, vegetable, and grain crops (specific crops claimed as sites differ between labels) * Also contains 5% ground garlic DENATONIUM SACCHARIDE (Ro-pel) Conc. Product Form Species 0.065%* Liquid Unspecified birds No. of Products 1 Use Considerations and Restrictions Taste repellent used to protect seeds or bulbs * Also contains 0.035% thymol LINDANE Conc. Product Form Species No. of Products Use Considerations and Restrictions 25.08% Powder Pheasants 1 Seed treatment for various agricultural crops 75.0% Powder Pheasants 1 Seed treatment for various agricultural crops * Also contains 12.5% captan and “related derivatives.” NAPHTHALENE Conc. Product Form Species 100% Flakes Unspecified birds No. of Products 1 Use Considerations and Restrictions Attics, floor and wall voids PETROLEUM NAPHTHENIC OILS Conc. Product Form Species No. of Products Use Considerations and Restrictions 79.65%* Aerosol Pigeons Starlings House sparrows 1 For treating ledges where birds roost or land 86.0%** Paste Pigeons Starlings “Nuisance birds” 1 For treating ledges where birds roost or land, and trees and shrubs * Also contains 1.65% polyisobutlyenes ** Also contains 2.0% polyisobutlyenes G-21 Table 22. (Continued) POLYBUTENES Conc. Product Form Species 42.8% Semi-liquid Starlings House sparrows Pigeons 1 For use on buildings and adjacent inanimate structures 49.0% Liquid Pigeons House sparrows Starlings Grackles Cowbirds Brewer’s blackbirds Common crows Ring-billed gulls Herring gulls California gulls 1 For interior use on beams, girders, struts, supports, and other places where birds roost or land 49.7% Paste Pigeons Starlings House sparrows Gulls 1 For treating ledges where birds roost or land 80% Paste Pigeons House sparrows Starlings Grackles Cowbirds Brewer’s blackbirds Common crows Ravens Ring-billed gulls Herring gulls California gulls 1 For use on structures where birds roost or land 90.0% Paste Pigeons 1 For use on buildings and adjacent inanimate structures 93.5% Paste Pigeons House sparrows 1 For treating structures where birds roost or land NOTE: No. of Products Use Considerations and Restrictions The heading “Polybutenes” is applied here to a variety of related compounds which are used to discourage birds from perching on treated areas due to the tackiness and other irritating tactile properties of these substances. Table 23. Pesticide federally registered to repel snakes. SULFUR Conc. Product Form Species 28.0%* Dry mixture Rattlesnakes Checkered garter snakes * Also contains 7.0% naphthalene Editors Scott E. Hygnstrom Robert M. Timm Gary E. Larson G-22 No. of Products 1 Use Considerations and Restrictions For use in areas around houses, cabins, trailers, garages, utility houses, barns, wood piles, sand piles, trash cans, and flower beds DESCRIPTION OF ACTIVE INGREDIENTS Compiled by Robert M. Timm The following pages contain information concerning the active ingredients commonly found in pesticides registered for control of wildlife damage. In general, each description follows a standard format that includes information about the compound’s use, history, physical and chemical properties, pharmacology, and toxicity. Toxicity is defined using the following abbreviations: For toxicity by oral exposure, LDL0 = lowest dose reported to be lethal to an animal LD50 = dose lethal to 50% of the animals tested LD100 = dose lethal to 100% of the animals tested MLD = minimum lethal dose, or LDL0. Doses are typically reported as mass of compound per mass of animal (mg/kg). For toxicity by inhalation, LCL0 = lowest concentration reported to be lethal to an animal LC50 = concentration lethal to 50% of the animals tested TCL0 = lowest concentration, for any given period of time, reported to produce any toxic effect. A toxicity expressed as LC50 = 300mg/m3/30M signifies that 50% of the population can be expected to die when exposed to a concentration of 300 mg per cubic meter of air for 30 minutes. For certain compounds, much of the descriptive information has been taken from the Vertebrate Pest Control Handbook (1986) as revised by Jerry P. Clark, and from the 1975 edition of the same publication compiled by Dell O. Clark. Both volumes were published by the California Department of Food and Agriculture, Sacramento. I thank Jerry Clark and Dell Clark for permission to use this material. Other references used are listed under “For Additional Information” following the description of many compounds, and under “References” at the end of this section. The publications by Spencer (1981), Sweet (1993), and Worthing (1991) were particularly helpful. Rex E. Marsh (University of California-Davis) and Kathleen Fagerstone (USDA-APHIS-Denver Wildlife Research Center) reviewed a draft of this section and provided many helpful comments, additions, and corrections. Additional review was provided by Bill Erickson (US-EPA); Ed Schafer, Jr., Peter Savarie, and Paul Woronecki (USDA-APHIS-Denver Wildlife Research Center); Russ Mason (Monell Chemical Senses Center, University of Pennsylvania); Scott Hygnstrom (University of Nebraska-Lincoln), and Gary Larson (USDA-APHISAnimal Damage Control). I greatly appreciate their help in improving the accuracy and usefulness of this section. PREVENTION AND CONTROL OF WILDLIFE DAMAGE — 1994 Cooperative Extension Division Institute of Agriculture and Natural Resources University of Nebraska - Lincoln United States Department of Agriculture Animal and Plant Health Inspection Service Animal Damage Control Great Plains Agricultural Council Wildlife Committee G-23 ACROLEIN Chemical Name Acrolein, 2-propenal, acrylaldehyde Trade Name Magnacide® Use Developed originally as an aquatic herbicide, it is useful in the control of submerged and floating weeds in freshwater ponds and other impoundments. History The original research leading to registration of acrolein as an aquatic herbicide was conducted by Shell Chemical Co., which patented the material in 1959 in the United States. Baker Performance Chemicals received a California Special Local Needs 24(c) registration for acrolein as a burrow fumigant for control of ground squirrels in 1993. Properties Acrolein is a colorless, highly volatile liquid with a pungent odor. It is moderately soluble in water at 20oC. It is miscible in the lower alcohols, ethers, hydrocarbons, acetone, and benzene. At room temperature, it is noncorrosive to iron or steel. At low doses, it irritates the throat and eyes, thus serving as its own warning agent. Toxicity Skin contact causes chemical burns in humans. A concentration of 1 ppm in air produces detectable eye and nose irritation in 2 to 3 minutes and is intolerable after 5 minutes. G-24 Oral LD50 values for acrolein have been reported as follows: Species Mouse Rat Rabbit Acute Oral LD50 (mg/kg)a 40 mg/kg 46 mg/kg 7 mg/kg Toxicity by inhalation has been reported as follows:a Species TCL0 LCL0 Mouse Rat Human LC50 66 ppm/6H 8 ppm/4Hb 300 mg/m3/30M 1 ppm aSweet (1993), unless otherwise noted bLewis and Sweet (1985) For Additional Information Lewis, R. L., and D. V. Sweet. 1985. Registry of Toxic Effects of Chemical Substances. 198384 Supplement. US Dep. Health and Human Serv., Public Health Serv., Centers for Disease Control, Nat. Inst. Occupational Safety and Health, Cincinnati, Ohio. O’Connell, R. A., and J. P. Clark. 1992. A study of acrolein as an experimental ground squirrel burrow fumigant. Proc. Vertebr. Pest Conf. 15:326-329. 153 ppm/10M ALUMINUM PHOSPHIDE Chemical Name Aluminum phosphide Trade Names Phostoxin®, Detia® Rotox®, Fumitoxin®, Gastoxin®, PhosTek® Use A fumigant for certain burrowing rodents and moles, it is also used to control insects in stored products. History Aluminum phosphide was introduced as a fumigant for stored products in the early 1930s by Dr. Werner Freyberg, Chemische Fabrik. Its formulation into molded tablets or pellets is a rather recent development. This material was registered for mammal control in 1981, although the compound has been used for this purpose in some other countries for a much longer time. Properties Aluminum phosphide forms dark gray or yellowish crystals. For mammal control, it is formulated into 3-g tablets or 600-mg pellets. A typical formulation contains 56% to 57% active ingredient plus 26% ammonium carbamate, 3% paraffin, and 14% to 15% aluminum oxide. Aluminum phosphide reacts with atmospheric moisture to release phosphine (PH3) gas, the active ingredient. Phosphine is colorless and has a slight carbide-like odor. At some concentrations it is flammable or explosive. In formulations which contain ammonium carbamate, this compound hydrolyzes to release CO2 and ammonia. Aluminum phosphide should be stored in its original metal container until used. Toxicity Phosphine gas is a potent mammalian toxicant. At a concentration of 1,000 ppm, it is lethal to humans after a few breaths. At 400 ppm, it is lethal in 30 minutes.a It is immediately dangerous to life or health at 200 ppm.b At a concentration of 1 ppm, it can be lethal to some rats within 24 hours.c The following toxicity values are given for phosphine gas: Species Inhalation LCL0d Mouse Cat Human 380 mg/m3/2H 70 mg/m3/2H 1000 ppm/5H For Additional Information Baker, R. O. 1992. Exposure of persons to phosphine gas from aluminum phosphide application to rodent burrows. Proc. Vertebr. Pest Conf. 15:312-321. Lewis, R. J., Sr., ed. 1979. Registry of toxic effects of chemical substances, 1978 ed. Nat. Inst. Occupational Safety Health, Centers for Disease Control, Public Health Serv., US Dep. Health, Education and Welfare, Cincinnati, Ohio. Salmon, T. P., W. P. Gorenzel, and W. J. Bentley. 1982. Aluminum phosphide (Phostoxin) as a burrow fumigant for ground squirrel control. Proc. Vertebr. Pest Conf. 10:143-146. aSpencer (1981) bBerg (1983) cLewis (1979) dSweet (1993) For complete citations, see References at the end of this section. G-25 ANTICOAGULANTS Chemical Names See below Trade Names See below Use Anticoagulants are a group of widely used rodenticides; an estimated 95% of all commensal rodent control is conducted with anticoagulants. They are separated into two functional groups, first-generation and second-generation anticoagulants. Those of the second generation have the ability to control warfarin-resistant rats and house mice, and they are also considered singlefeeding anticoagulants. First generation anticoagulants are also used for the control of certain field rodents, including ground squirrels, pocket gophers, and voles. Some field rodent and rabbit registrations are specific to local needs of various states, and they are extensively used to protect agricultural crops and forest trees. At present, none of the second generation anticoagulants are registered for control of field rodents or rabbits. History Warfarin, the first anticoagulant rodenticide, had its beginning in 1943 when Dr. Karl Paul Link and his co-workers of the Biochemistry Department, University of Wisconsin, were attempting to determine the cause of “Sweet Clover Disease” in cattle. Moldy sweet clover hay was found to contain a powerful anticoagulant. The first result of the research was the development of dicumarol, which is used to prevent the formation of blood clots in humans. Dr. Link’s staff continued the line of research and synthesized warfarin (Compound 42) which is a much more potent anticoagulant than dicumarol. In April 1948, J. A. O’Connor described the first successful use of an anticoagulant compound, dicoumarin, for controlling rats under field conditions. G-26 Pindone, coumafuryl, and valone soon followed warfarin on the market, with diphacinone and chlorophacinone marketed somewhat later. The last two compounds were, by far, more toxic than the earlier materials; hence, the concentration in baits was reduced by some fivefold. Of the earlier anticoagulants, coumafuryl (Fumarin®) and valone (PMP®) are no longer marketed. The second generation anticoagulants, bromadiolone and brodifacoum, were developed some years later specifically to combat warfarin resistance. The newest of the second-generation anticoagulants, difethialone, has been in development for a number of years and is nearing registration in the United States. Characteristics Anticoagulants used as rodenticides are chemically separated into two general groups: the hydroxycoumarins (such as warfarin) and the indandiones (pindone, valone, diphacinone, and chlorophacinone). The second generation materials (bromadiolone, brodifacoum, and difethialone) are closely akin to the hydroxycoumarin group. Table 1 lists the anticoagulants in current use or about to be registered in the United States. All first-generation anticoagulants, also known as multiple-dose rodenticides, relied on their cumulative toxic effect. They were substantially more toxic if consumed in small doses over a period of several days than if consumed in one large amount (for instance, the 5-day cumulative LD50 is substantially lower than the acute LD50). The baits are formulated so that rodents have to feed a minimum of 3 to 5 days before a lethal dose is achieved; death follows after several additional days. In order to achieve this multiple feeding, the bait must be made available on a continuous basis until the desired control is reached. Prior to the development of anticoagulants, all rodenticides were acute (single dose) materials; hence, the introduction of warfarin required a whole new concept of bait application. Bait trays or bait boxes had to be designed to hold substantial amounts of bait and strategically located so that all rodents in an area had access to ample bait for repeated feedings until death. Bromadiolone, brodifacoum, and difethialone, all second generation materials, are much more potent, with relatively low acute LD50s for rodents, making them effective for the control of warfarin-resistant rats and mice. When formulated at their current concentrations, they have the ability to kill a high percentage of the rodent population in a single feeding, hence their designation as single-feeding anticoagulants. The effects of these compounds are also cumulative and will result in death after several feedings of even small amounts. As in the case of all anticoagulants, death is delayed for several days following the ingestion of a lethal dose. This delayed action has a decided safety advantage because it provides time to administer the antidote and save pets, livestock, and of course, people who may have accidentally ingested the bait. Vitamin K1 is the antidote for anticoagulants and, if administered soon enough after intake, can reverse the action of the anticoagulant. Diphacinone, chlorophacinone, and all of the second-generation materials Table 1. Anticoagulants used in the United States. Chemical Name Usual Types of Formulations Food Tracking Bait Liquid Powder Percent Active Ingredient Used in Food Bait 3-(α-acetonylbenzyl)-4-hydroxycoumarin X 0.025 3-[3(4'-bromo[1,1’biphenyl]-4-yl)-1,2,3,4-tetrahydro1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one X Bromadiolone (Maki® Contrac®)* 3-[3-(4'-bromo[1,1’biphenyl]-4-yl)-3-hydroxy-1phenylpropyl]-4-hydroxy-2H-1-benzopyran-2-one X 0.005 Difethialone* [(bromo-4’-[biphenyl-1-1’]-yl-4)3-tetrahydro-1,2,3,4napthyl-1]3-hydroxy-4,2H-1 benzo-thiopyran-2-one X 0.0025 Chlorophacinone (RoZol®) 2-[(p-chlorophenyl)phenylacetyl]-1,3-indandione X X 0.005 Diphacinone (Ramik®, Contrax-D®) 2-diphenylacetyl-1,3-indandione X X 0.005 Pindone (Pival®, Pivalyn®, Contrax-P®) 2-pivalyl-1,3-indandione X Common Name and Typical Trade Names Hydroxycoumarins Warfarin (d-Con and others) Brodifacoum (Talon®, Havoc®)* X 0.005 Indandiones X 0.025 *Second-generation anticoagulants especially useful for the control of warfarin-resistant rats and mice. persist in animals and will often require prolonged veterinary or medical treatment. The slow action of anticoagulant baits has another great advantage in that the target animal is unable to associate its illness with the bait eaten. Therefore, bait shyness or toxicant shyness does not occur. Most of the anticoagulant baits used today are commercial ready-to-use baits; very few individuals prepare their own baits from concentrates as they commonly did 20 years ago. Ready-to-use bait increases the cost of rodent control but avoids past problems of incorrect bait concentrations and poor bait formulation, which often led to poor control. Some anticoagulants are available as tracking powders and others as sodium salts that are water-soluble, allowing their use as water baits. In the early 1960s the practice began of mixing anticoagulant grain baits with melted paraffin and molding it into cans or cartons to form block-type paraffin baits. These became commercially available a few years later and were promoted for sewer rat control or for other rodent-infested areas with moisture and high humidity. Now there are molded or extruded paraffin-type baits made from most of the current anticoagulants. Block-type baits have several advantages: they confine multiple feedings of bait into one unit; if permitted by the label, they can be placed in strategic locations where bait boxes with loose grain or pelleted bait would be difficult to place; and bait deterioration from insects and molds is retarded. Anticoagulant Resistance The resistance of rats to warfarin was first noted in Scotland in 1958, some years following its repeated use. Shortly thereafter, anticoagulant resistance was identified in both rats and house mice in other European countries. It was identified somewhat later in the United States, where it has since been demonstrated in many regions and major cities. All three species of commensal rodents are implicated. Resistance has not been found in field rodents. Resistance arises from genetic mutation or recombination, sometimes of a single gene, and levels of resistance vary among individual animals. A high degree of resistance will render control with warfarin virtually impossible. Rats and mice that are resistant to warfarin also show some resistance to all first generation anticoagulants. Where resistance is apparent, switch to a second generation anticoagulant or to another rodenticide with a different mode of action. Whether resistance will eventually extend to all second-generation anticoagulants remains to be seen; some isolated instances of resistance to bromadiolone have been reported. Pharmacology All anticoagulants have two actions; they reduce the clotting ability of the blood and cause damage to the capillaries (tiny blood vessels). The rate of blood clotting gradually decreases and blood loss leads to an apparently painless death. Animals killed by anticoagulants often have no color in the skin, muscles, or viscera. Evidence of hemorrhage may be found in any part of the body, but usually only in one location. The blood that remains in the heart and vessels is very thin and forms a poor clot or no clot. The animal exhibits increasing weakness though appetite and body weight are not specifically affected. Hematoma (a local swelling or tumor filled with blood) formation beneath the skin is often more common than free hemorrhage. G-27 Repeated daily doses of the anticoagulants greatly increases their effective toxicity. Feeding does not have to be on consecutive days, but several feedings should occur within a 10-day interval with no longer than 48 hours between feedings. Plenty of bait must be made available at all times to achieve adequate control. Toxicity The susceptibility to anticoagulants varies considerably among species and among anticoagulants. For this reason, generalizing often leads to erroneous conclusions. Since all anticoagulants are cumulative in toxicity, they have the ability to kill any warm-blooded animal if consumed in sufficient amounts for a long enough period. Materials with the highest toxicity and the longest half-lives present the greatest lethal potential with fewer feedings. Compounds with the longest half-lives need not be consumed daily; a lapse of several days between feedings will not alter the outcome. Many drugs increase the effects of anticoagulants; among these are the broad-spectrum antibiotics, the barbiturates, and the salicylates. Observations of rats treated with chlordane and DDT show the opposite effect; they stimulate the metabolism of warfarin, thus decreasing its toxicity. Susceptibility to anticoagulants seems to increase with age. Anticoagulants tend to accumulate in the liver and gradually dissipate over a period of time, depending on the initial accumulations and successive does. Where large doses of anticoagulants are ingested, substantial amounts may pass through the animal unassimilated. Precautions should be taken to prevent children, pets, and livestock from gaining access directly to anticoagulant bait. Baits should be placed in areas inaccessible to nontarget animals or in tamper-resistant bait stations. A single substantial ingestion of diphacinone, chlorophacinone, or any of the secondgeneration anticoagulant baits may, for example, place a dog in jeopardy, requiring veterinary attention. When G-28 used according to label instructions, there is little potential hazard to nontarget species. Secondary hazard associated with predator or scavenger animals consuming rodent carcasses is minimal in commensal rodent control. It can be of somewhat greater concern when anticoagulants are used for field rodent control. Occasionally a farm dog is known to consume fresh vole or ground squirrel carcasses over several days and begin to show signs of anticoagulant intoxication. With quick and proper veterinary attention, the dog can usually be saved. Although secondary poisoning has been demon- strated in the laboratory for various species, its occurrence in the wild appears very low, with few documented cases where use recommendations were followed. Toxicity information for many animals is not readily available. A man with suicidal intent induced serious illness by ingesting 1.7 mg warfarin per kg per day for 6 consecutive days. This corresponds to eating almost 1 pound of bait (0.025% warfarin) per day. All signs and symptoms were caused by hemorrhage, and following multiple small transfusions and massive doses of vitamin K, recovery was complete.a The following toxicity data have been reported: Brodifacoum Species House mouse Norway rat Roof rat Meadow vole Pine vole Rabbit Pig Dog Cat Chicken Bromadiolone Species House mouse, albino Norway rat, albino Rabbit Chlorophacinone Species Vampire bat House mouse White (lab) rat, on meat Norway rat, albino Roof rat Deer mouse Rabbit Mallard Ring-necked pheasant Red-winged blackbird Acute Oral LD50 (mg/kg)b 0.4 - 0.86 0.27 0.65 - 0.73c 0.72d 0.36d 0.29 0.5 - 2.0 0.25 - 1.0 ~25 10 - 100 Acute Oral LD50 (mg/kg)d,e 1.75 1.125 1.0 LD50 (mg/kg) (presumably from single oral dose)a 7.5 1.06d 2.1 2.1 - 20.5d 15.0 0.49 50d >100 >100 430 Difethialone Acute Oral LD50 (mg/kg)f Species House mouse (wild) Norway rat (wild) Roof rat (wild) Pig Bobwhite quail Diphacinone Species House mouse Mouse Rat Rat Rabbit Dog Coyote Cat Pig Pindone Species Acute Oral LD50 (mg/kg) 141 - 340d 340g 1.9d - ~3h 3 - 17g 35k 0.8 - 15d,h,i 0.6 j 5 - 15d 150h Acute Oral LD50 (mg/kg) Species Chronic Oral LD50 (mg/kg/day) (lab rat) 0.1h 0.25 for 14 daysd Chronic Oral LD50 (mg/kg/day) 0.52 for 7 daysd 2.5h Acute Oral LD50 (mg/kg) Mouse Rat Rat Rabbit Swine Dog Cat Cat Ruminants Chicken 374d 3.0d 50-100l 800d 3l d 20 - 50l 6 - 40d 5 - 50l 1,000d aClark (1986) Dubock, A. C., and D. E. Kaukeinen. 1978. Brodifacoum (Talon rodenticide), a novel concept. Proc. Vertebr. Pest Conf. 8:127-137. Greaves, J. H. 1985. The present status of resistance to anticoagulants. Acta Zool. Fennica 173:159-162. 280d 50d Rat Rat, albino Rabbit Dog Warfarin 0.47 0.51 0.38 2-3 0.264 For Additional Information Chronic Oral LD50 (mg/kg/day) daysa 0.6 for 3 - 9 0.4 for 4 - 15 daysa 1 for 5 daysl 30.0 for 6 - 15 daysa 0.05 for 7 daysl 5 for 5 - 15 daysl 3.0 - 5.0 for 10 daysa 1 for 5 daysl 200 for 12 daysl Hadler, M. R., and A.P. Buckle. 1992. Forty-five years of anticoagulant rodenticides—past, present and future trends. Proc. Vertebr. Pest Conf. 15:149-155. Kaukeinen, D. 1982. A review of the secondary poisoning hazard potential to wildlife from the use of anticoagulant rodenticides. Proc. Vertebr. Pest Conf. 10:151-158. Kaukeinen, D. E., and M. Rampaud. 1986. A review of brodifacoum efficacy in the U.S. and worldwide. Proc. Vertebr. Pest Conf. 12:16-50. Lechevin, J. C., and R. M. Poché. 1988. Activity of LM2219 (difethialone), a new anticoagulant rodenticide, in commensal rodents. Proc. Vertebr. Pest Conf. 13:59-63. Murphy, M. J., and D. F. Gerken. 1989. The anticoagulant rodenticides. Pages 143-146 in R. W. Kirk, ed. Current veterinary therapy X: small animal practice. W. B. Saunders Co., Philadelphia. Poché, R. M. 1986. The status of bromadiolone in the United States. Proc. Vertebr. Pest Conf. 12:6-15. Richards, C. G. J., and L. W. Huson. 1985. Towards the optimal use of anticoagulant rodenticides. Acta Zool. Fennica 173:155-157. Savarie, P. J., D. J. Hayes, R. T. McBride, and J. D. Roberts. 1979. Efficacy and safety of diphacinone as a predacide. Pages 69-79 in E. E. Kenaga, ed. Avian and mammalian wildlife toxicology. ASTM Spec. Tech. Pub. 693. Am. Soc. Testing Materials, Philadelphia. Woody, B. J. 1992. Clinical approach to the diagnosis of diseases and disorders in pets and domestic animals sometimes mistaken for anticoagulant toxicosis. Proc. Vertebr. Pest Conf. 15:199-203. bTalon Technical Bulletin, ICI Americas, Inc., (1987) cDubock and Kaukeinen (1978) dHone and Mulligan (1982) eMaki Technical Bulletin, Chempar Chemical Co. Inc. (no date) fLechevin and Poché (1988) gHumphreys (1988) hSpencer (1981) iTechnical Bulletin, Velsicol Chemical Co. (1977) jSavarie et al. (1979) kHumphreys (1988) lOsweiler et al. (1985) For complete citations, see References at the end of this section. G-29 AVITROL® Chemical Name 4-aminopyridine Trade Name Avitrol® Use Avitrol® is a bird management chemical registered for use as a flockfrightening repellent. It is usually formulated as a grain bait. Treated bait is diluted with untreated bait so that only a few birds in a flock ingest a treated particle of bait. Affected birds emit distress cries and/or perform visual displays that often frighten the other birds in the flock, causing them to leave. Avitrol® has been used for feral pigeons, house sparrows, and for certain blackbirds and cowbirds in and around structures. In agricultural situations, crows, starlings, grackles, cowbirds, and blackbirds are most frequently the targeted species. Avitrol® products are for use by or under the supervision of government agencies or certified control operators. Avitrol® is not for sale to the public. History Avitrol® is the registered trademark of the Avitrol Corporation for the chemical 4-aminopyridine. The synthesis of G-30 this chemical was first reported in 1931, and its unique action on birds was reported in 1964 by Goodhue. Its utility for controlling damage by birds in some situations was demonstrated in 1965 by Goodhue and Baumgartner. Characteristics 4-aminopyridine is a white crystalline, odorless, water-soluble material. It is stable in light and melts at 159oC. Pharmacology Avitrol® is an acutely toxic substituted pyridine that affects the nervous system in a manner similar to that of organophosphates and carbamates; however, Avitrol® is not a cholinesterase inhibitor. In most bird species, a lethal dose of Avitrol® is necessary to produce distress behavior. Toxicity Birds and mammals appear equally sensitive to Avitrol® intoxication. LD50 values are generally less than 10 mg/kg. Birds ingesting the material become disoriented, emit distress calls, and exhibit erratic flight, tremors, and convulsions before death. Distress usually begins in about 15 minutes and lasts 20 to 30 minutes in most species. Some species, such as pigeons, do not emit distress calls. In mammals, the following symptoms are produced: hyperexcitability, salivation, tremors, muscular incoordination, convulsions, cardiac or respiratory arrest, and death. Initial effects are usually noted in 10 to 15 minutes and death often occurs 15 minutes to 4 hours later. Occasionally the tremor and/or convulsive stages are accompanied by audible vocalizations produced by strong, involuntary contractions of the diaphragm. Documented reports of secondary poisoning following Avitrol® use have been very limited. When birds are offered undiluted Avitrol® baits, there may be potential hazards to dogs, cats, and raptors that consume unassimilated Avitrol® in gut contents. In field use, only individual scavengers such as magpies and crows appear to be have been impacted. Toxicity Table for Avitrol®a Species For Additional Information Acute Oral LD50 (mg/kg)a,b Acute Oral LD50 (mg/kg) for HCl* a,b 20 28 - 32.5 17.8 11.9 MAMMALS White rat Hog Dog 3.7 - 4.0 BIRDS Mallard American kestrel Domestic chicken (2-3 wk. old) Coturnix quail Ring-necked pheasant (4 wk. old) Ring-billed gull Common pigeon White-winged dove Mourning dove American robin Starling Black-billed magpie Common crow Yellow-billed magpie Boat-tailed grackle Brown-headed cowbird Common grackle Red-winged blackbird Shiny cowbird Tricolored blackbird House finch Golden-crowned sparrow House sparrow White-crowned sparrow 4.22 5.62 15 7.65 - 8.05 5.62 - 7.50 Besser, J. F., D. J. Brady, T. L. Burst, and T. P. Funderberg. 1984. 4-Aminopyridine baits on baiting lanes protect sunflower fields from blackbirds. Agric. Ecosystems and Environ. 11:281-290. Schafer, E. W., Jr., R. B. Brunton, and D. J. Cunningham. 1973. A summary of the acute toxicity of 4-aminopyridine to birds and mammals. Toxicol. Appl. Pharmacol. 26:532538. Schafer, E. W., Jr., W. A. Bowles, Jr., and J. Hurlbut. 1983. The acute oral toxicity, repellency and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environ. Contam. Toxicol. 12:355-382. 8 20 7.5 8.10 - 8.50 4.22 4.90 - 6.0 2.37 2.37 2.37 1.7 - 7.1 4.22 2.37 1.78 - 8.50 < 1.00 4.22 5.62 5.62 3.00 - 7.50 5.62 14 3.2 * HCl = Hydrochloride salt of 4-aminopyridine. aSchafer et al. (1973) bSchafer et al. (1983) For complete citations, see References at the end of this section. G-31 BONE TAR OIL Trade Name Magic Circle Deer Repellent® Use As an area (odor) repellent to protect trees, shrubs, and other plant materials from deer. History Developed as a deer repellent at State College Laboratories, Pennsylvania, during the 1950s. Magic Circle Deer G-32 Repellent® was registered and marketed by State College Laboratories, a division of J. C. Ehrlich Chemical Company until 1993. Properties The active ingredient, bone tar oil (also called bone oil), is a dark, viscous liquid with a distinct odor. It is produced by the destructive distillation of animal bones. The commercial product contains 93.75% active ingredient. Toxicity The human oral LDLO is 500 mg/kg. The oral LDL0 for rats is 800 mg/kg.a aSweet (1993) For complete citations, see References at the end of this section. BROMETHALIN Chemical Name N-methyl-2,4-dinitro-N-(2,4,6-tribromophenyl)-6-(trifluoromethyl) benzenamine Other Name Bromethalin Trade Names Assault®, Vengeance®, Trounce® Use A single-dose rodenticide developed for the control of Norway rats, roof rats, and house mice. History The toxic nature of certain classes of diphenylamines was researched by Eli Lilly Research Laboratories in the 1970s. Laboratory and field research on bromethalin progressed into the early 1980s, at which time it was registered by EPA. Properties Bromethalin is a pale yellow, odorless crystalline solid. Its melting point is 151oC. Bromethalin is insoluble in water but is soluble in many organic solvents. are reversible if administration of the toxicant is discontinued. Experiments in the physiological and biochemical mechanisms of action suggest that bromethalin uncouples oxidative phosphorylation in central nervous system mitochondria. This could lead to a decreased production of ATP, a diminished activity of Na + /K + ATP-ase, and a subsequent fluid buildup manifested by fluid-filled vacuoles between the myelin sheaths. This vacuole formation in turn leads to an increased cerebrospinal fluid pressure and increased pressure on nerve axons, yielding a decrease in nerve impulse conduction, paralysis, and death. No secondary hazard is known. Rodents consuming a sublethal dose are not reported to become bait shy. Pharmacology Both acute and chronic effects can occur following ingestion. Acute effects include tremors, one or two episodes of clonic convulsions, prostration, and death usually within 18 hours. These effects occur when technical bromethalin is administered in a soluble form and given at a dose two-fold or greater than the LD50, or by generous bait consumption. Chronic effects, which include lethargy, hind-leg weakness, loss of muscle tone and paralysis, occur with a single dose equal to the LD50, with multiple smaller doses, or with sublethal dietary administration. Several acute and dietary studies have shown that, at sublethal doses, these effects Toxicity The toxicity of bromethalin is as follows: Species Laboratory mouse Laboratory rat (Norway) Roof rat Rabbit Dog Cat Monkey Adult quail Acute Oral LD50 (mg/kg)a 5.25 - 8.13 Bait placement and timing are crucial in achieving effective rodent control with bromethalin bait. Bait should be renewed at intervals of several days. Continuous bait availability (as with anticoagulants) is not required, but bait needs to be exposed long enough to allow all animals in the area to feed. Bait quantity will be about one-third that used with anticoagulants, since an animal ingesting a lethal dose does not feed again. For Additional Information Dorman, D. C. 1992. Bromethalin rodenticide toxicosis. Pages 175-178 in R. W. Kirk and J. D. Bonagura, eds. Current veterinary therapy XI: small animal practice. W. B. Saunders Co., Philadelphia. Jackson, W. B., S. R., Spaulding, R. B. L. Van Lier, and B. A. Dreikorn. 1982. Bromethalin—a promising new rodenticide. Proc. Vertebr. Pest Conf. 10:10-16. Spaulding, S. R., and H. Spannring. 1988. Status of bromethalin outside the United States. Proc. Vertebr. Pest Conf. 13:64-69. Van Lier, R. B. L., and L. D. Ottosen. 1981. Studies on the mechanism of toxicity of bromethalin, a new rodenticide. Toxicol. 1(1):114. 2.01 - 2.46 6.6 13.0 4.7 1.8 5.0 4.6 Chronic Oral LD50a Quail Mallard 210 ppm 620 ppm aJackson et al. (1982) G-33 CAPSAICIN Chemical Name 8-methyl-n-vanillyl-6-nonenamide Trade Name Hot Sauce Animal Repellent® Use Properties Toxicity Capsaicin is the material that makes peppers (red, jalapeño, and others) hot. It is also the active ingredient in a repellent used to protect ornamental trees and shrubs, dormant fruit and nut trees, and nursery stock. It is registered for controlling damage by deer, rabbits, and meadow and pine mice. It is also registered as an active ingredient in several dog repellents (Halt®, Dog Shield®) and is frequently used by mail carriers and meter readers to ward off aggressive dogs. It has recently been proposed for federal registration to repel attacking bears and moose. Capsaicin (from oleoresin of capsicum) is a liquid with a boiling point of 220oC. It is soluble in vegetable oil and in various solvents. The repellent formulation is a brown to reddish homogeneous liquid at room temperature, containing natural fats and waxes. The acute oral LD50 for the mouse is reported to be 47.2 mg/kg.a History Developed as a repellent by Miller Chemical and Fertilizer Corporation, capsaicin was federally registered by the EPA in 1980. G-34 Pharmacology Capsaicin is an extreme irritant to skin, eyes, and other tissues. Exposure to skin causes dermal irritation. Exposure to eyes can cause superficial keratitis and conjunctivitis. Ingestion can cause severe irritation to the stomach or lungs. When handling the concentrate, use rubber or plastic gloves and a face shield. aSweet (1993) For Additional Information Mason, J. R., N. J. Bean, P. S. Shah, and L. Clark. 1991. Taxon-specific differences in responsiveness to capsaicin and several analogues: correlates between chemical structure and behavioral aversiveness. J. Chem. Ecol. 17(12):2539-2551. Rogers, L. L. 1984. Reactions of free-ranging black bears to capsaicin spray repellents. Wildl. Soc. Bull. 12:59-61. ALPHA-CHLORALOSE Chemical Name 1,2-0-(2,2,2-trichloroethylidene)-α-D-glucofuranose Other Names chloralose, glucochloralose, A-C Trade Names Alphakil®, Murex, Somio Use This compound has recently been approved in the United States for use as an immobilizing agent for waterfowl, coots, and pigeons. It is not classified as a pesticide, but rather as a drug. Federal approval for its use by USDA-APHIS personnel and their trained designees was granted by the US Food and Drug Administration in 1992. History Alpha-chloralose has been used in baits for stupefying pigeons and house sparrows in the United Kingdom since 1959. It has also been employed in colder climates of European countries as a rodenticide. It has been formulated at about 0.5% in grain baits for birds, but up to 4% active ingredient as a rodenticide against house mice.a The compound has also been used as a general anesthetic in laboratory animals, and as an activating agent in electroencephalography in humans. Properties This compound is a crystalline powder with a melting point of 187oC. It is soluble in ether and in glacial acetic acid. Its solubility in water is 1 g in 225 ml at 15oC. Pharmacology Alpha-chloralose is a centrally active drug with both stimulant and depressant properties on the central nervous system. In birds, alpha-chloralose acts as a hypnotic, rendering birds easier to capture. In rodents, it retards metabolism, lowering body temperature to a degree that may be fatal to small mammals such as house mice in cold climates (below 15oC.). In mice, ataxia occurs in 5 to 10 minutes following first ingestion, and feeding ceases within 20 minutes. Mice may recover at air temperatures above 15oC. Birds will recover if left alone.d In small doses, the compound increases motor activity and causes myoclonic movements, which may progress eventually to deep anesthesia as dosage increases. It selectively depresses the normal arousal response. The electroencephalogram (EEG) of anesthetized animals, however, suggests that cortical neurons are in a “subconvulsive state.”a Alpha-chloralose is metabolized to chloral, which is converted to trichlorethanol, a central nervous system depressant. Hepatic formation of a glucuronide then occurs to form pharmacologically inactive urochloralic acid, which is excreted in the urine.b Early clinical effects are mild ataxia followed by hyperexcitability. Some cats may become quite aggressive. In severe poisoning, the early signs rapidly give way to posterior weakness, prostration, increased salivation, shallow respiration, weak pulse, and hypothermia. Affected animals appear to lose sensitivity to pain but have increased reactivity to touch, sound, or electrical stimulus.c A sort of “psychic blindness” is described in dogsb, in which affected animals do not respond to normal stimuli or familiar surroundings. Fatally poisoned animals appear to die of respiratory failure.c Toxicity Birds are generally less resistant than mammals to alpha-chloralose. Under experimental conditions, cats have refused to voluntarily consume baits containing the compound, while dogs have eaten 4 g/kg without lethal effects.b The following toxicity values have been reported: Acute Oral LD50 (mg/kg) Species House mouse Norway rat Cat Goose, canada Mallard Coot Pigeon Crow Starling 190d - 300e 300d - 400e,f 100e - 250f 54g 42d,f 47 - 58h 178d,f - 215g 42e 75e aSpencer (1981) bLees and Pharm (1972). cHayes (1982) dHone and Mulligan (1982) eOsweiler et al. (1985) fSweet (1993) gWoronecki (1992) hWoronecki, unpubl. For complete citations, see References at the end of this section. For Additional Information Cornwell, P. B. 1969. Alphakil—a new rodenticide for mouse control. Pharmaceut. J. 202:74-75. Lees, P., and Y. Pharm. 1972. Pharmacology and toxicology of alpha-chloralose: a review. Vet. Rec. 91:330. Woronecki, P. P., R. A. Dolbeer, and T. W. Seamans. 1990. Use of alpha-chloralose to remove waterfowl from nuisance and damage situations. Proc. Vertebr. Pest Conf. 14:343-349. Woronecki, P. P., R. A. Dolbeer, and T. W. Seamans. 1992. Alpha-chloralose efficacy in capturing nuisance waterfowl and pigeons and current status of FDA registration. Proc. Vertebr. Pest Conf. 15:72-78. G-35 CHLOROPICRIN Chemical Names Chloropicrin, trichloronitromethane, nitrochloroform Trade Names Larvacide®, Chlor-o-pic®, and others Use Toxicity For Additional Information Chloropicrin is a fumigant for controlling rats and mice in structures or burrows. It has been found to be an effective area repellent for house mice. It is also used as a fumigant for insect control in soil and stored grain and cereal products. Acute oral LD50 for Norway rats is 250 mg/kg.b The following toxicities for inhalation of the compound have been reported: Tigner, J. R., and W. A. Bowles. 1964. Chloropicrin tested as an area repellent for house mice. J. Wildl. Manage. 28:748-751. History Its first use as an insecticide was suggested in 1908. It was widely used in chemical warfare (tear gas) from 1916 to 1918. Properties Chloropicrin is a heavy colorless liquid with a boiling point of 112oC. It is not flammable. It is soluble in water and miscible with various organic solvents. Chloropicrin is easily detected at very low concentrations because of its characteristic odor and tear gas effect. Hence, it is used as a warning agent in other fumigants. Because of this effect, it may drive rodents out of structures or burrows before they receive a lethal amount. Pharmacology The compound is very irritating to mucous membranes, causing tear production. Chloropicrin injures the medium and small bronchi. Pulmonary edema is the cause of death.a Because of its irritating properties, it is not usually lethal to humans as long as an escape route exists. G-36 Species LCL0 Mouse Human 2,000 mg/m3/Mb aOsweiler LC50 66 mg/m3/4Hb (1985) bSweet (1993) For complete citations, see References at the end of this section. CHOLECALCIFEROL Chemical Name 9,10-seocholesta-5,7,10(19)-trein-3 betaol Other Names Cholecalciferol, Vitamin D3 Trade Names Quintox®, Rampage® Use Cholecalciferol is a single-dose or multiple-dose rodenticide. History Laboratory and field research of this compound as a rodenticide was conducted relatively recently, although rodenticidal properties of calciferol, a related compound, have been known for some time. Registration as a rat and mouse bait was granted to Bell Laboratories in late 1984 and Motomco Ltd. in early 1985. Properties In pure form, cholecalciferol is a light brown resin with a melting point of 84o to 85oC. It is not soluble in water but will dissolve in some organic solvents. Pharmacology Cholecalciferol is metabolized in the liver to 25-hydroxycholecalciferol and then in the kidney to 1-alpha, 25dihydroxycholcalciferol. It causes mobilization of calcium from the bone matrix to plasma. Victims die from hypercalcemia. Time to death is 3 to 4 days after receiving a lethal dose. Bait shyness is said not to occur. Once a rodent consumes a lethal dose, all food intake is claimed to cease. This effect is unlike that of anticoagulants, in which rodents continue to consume bait after they have ingested a lethal dose. Toxicity Originally, the acute oral LD50 for laboratory mice was reported as 42.5 mg/kg; for laboratory rats, 43.6 mg/ kg.a For dogs, the reported acute oral LD50 was 80 mg/kg.b More recent information based on use of formulated products suggests the LD50 values for rats and for dogs are in the range of 10 to 15 mg/kgc, or perhaps lower. Dogs and cats are more susceptible to this compound than are some rodents, and precautions must be taken to prevent their direct access to the bait. Secondary toxicity from feeding on poisoned rodents has not been demonstrated. For Additional Information Beard, M. L., G. O. Maupin, A. M. Barnes, and E. F. Marshall. 1988. Laboratory trials of cholecalciferol against Spermophilus variegatus (rock squirrels), a source of human plague (Yersinia pestis) in the southwestern United States. J. Environ. Health 50(5):287-289. Brown, D. L., and E. F. Marshall. 1988. Field evaluation of Quintox® (cholecalciferol) for controlling commensal rodents. Proc. Vertebr. Pest Conf. 13:70-74. Dorman, D. C., and V. R. Beasley. 1989. Diagnosis of and therapy for cholecalciferol toxicosis. Pages 148-152 in R. W. Kirk, ed. Current veterinary therapy X: small animal practice. W. B. Saunders Co., Philadelphia. Livezey, K. L., D. C. Dorman, S. B. Hooser, and W.B. Buck. 1991. Hypercalcemia induced by vitamin D3 toxicosis in two dogs. Canine Practice 16(5):26-32. Twigg, L. E., and B. J. Kay. 1992. Evaluation of Quintox® for control of feral house mice. J. Wildl. Manage. 56:174-185. aTechnical Release, Bell Laboratories Inc. (1983) bSweet (1993) cPersonal communication, William Erickson G-37 DENATONIUM SACCHARIDE Chemical Name Benzyldiethyl ([2,6-xylylcarbamoyl]methyl) ammonium saccharide Trade Name Ro-pel® Use Ro-pel® is a repellent in a liquid formulation, for application to surfaces to prevent animal feeding or gnawing. Ro-pel® is currently registered for use against deer, beaver, rats, mice, tree squirrels, birds, dogs, and cats. The formulated product contains as active ingredients 0.065% denatonium saccharide and 0.035% thymol. Ro-pel® is described to be “extremely bitter and vile tasting.” History Denatonium saccharide was developed in the 1980s as a chemical agent by Burlington Scientific for the US government, for potential use in making food crops inedible or rendering drug-producing plants useless. G-38 It is chemically related to denatonium benzoate (trade name: Bitrex®), another bitter-tasting compound which is currently registered as a dog and cat repellent, and which has recently been incorporated into some commensal rodent baits as a safety precaution to prevent their ingestion by children. Toxicity No toxicity data for denatonium saccharide is available, although the toxicity appears to be low to both birds and mammals. For denatonium benzoate (Bitrex®), the acute oral LD50 for the rat is 584 mg/kg, and for the rabbit, 508 mg/kg.a aSweet (1993) For Additional Information Davis, S. F., C. A. Grover, C. A. Erickson, L. A. Millere, and J. A. Bowman. 1987. Analyzing aversiveness of denatonium saccharide and quinine in rats. Perceptual Motor Skills 64:1215-1222. Kaukeinen, D. E., and A. P. Buckle. 1992. Evaluations of aversive agents to increase the selectivity of rodenticides, with emphasis on denatonium benzoate (Bitrex®) bittering agent. Proc. Vertebr. Pest Conf. 15:192-198. Payne, H. A. 1988. Bitrex—a bitter solution to safety. Chem. Ind. Nov. 21 issue. pp. 721-723. EGG SOLIDS, PUTRESCENT Trade Names Deer-Away® Use A repellent for protecting conifers, ornamental trees and shrubs, nonbearing fruit trees, and bearing fruit trees before flowering and leafing out and after harvest, from deer and elk. History Developed by researchers at the Weyerhaeuser Company during the 1970s, and later manufactured and marketed by McLaughlin Gormley King Company. Deer-Away® is currently marketed by IntAgra, Inc. Properties The product is formulated as a concentrate for making a liquid solution, and as a powder. The concentrate consists of two parts. Concentrate 2103 is a brownish paste-like slurry with a slight fermented egg odor. It contains 37% putrescent whole egg solids and 63% inert ingredients. Formula 2104 is a red liquid that contains only inert ingredients. The concentrates have a shelf life of more than 1 year. When combined and diluted, they result in a ready-touse spray. For Additional Information Toxicity Palmer, W. L., R. G. Wingard, and J. L. George. 1983. Evaluation of white-tailed deer repellents. Wildl. Soc. Bull. 11:164-166. A repellent concentrate containing 15% putrescent whole egg solids and 85% inert ingredients was found to have an acute oral LD50 for rats of 34,600 g/kg. It had an acute dermal LD50 for rabbits of 3,000 mg/kg. No apparent acute or chronic effects were seen in penned deer exposed to the product for several years.a Andelt, W. F., K. P. Brunham, and J. A. Manning. 1991. Relative effectiveness of repellents for reducing mule deer damage. J. Wildlife Manage. 55:341-347. Conover, M. R. 1984. Effectiveness of repellents in reducing deer damage in nurseries. Wildl. Soc. Bull. 12:399-404. The product may cause browning and drop of old needles on some species of conifers. aTechnical Report, Deer-Away (no date) G-39 FATTY ACIDS (various compounds) Chemical Names a) Ammonium soaps of higher fatty acids b) Sodium salts of mixed fatty acids Trade Names a) Hinder®, Repel b) Bye Deer® Use History Properties Repellents for protection of various plants from animal feeding. Hinder® is registered as a deer and rabbit repellents for use on fruit trees and vines, vegetables and field crops, ornamentals, nursery stock, forage crops, grain crops, and noncrop areas. This product contains 15% active ingredient. Label directions instruct dilution with water prior to application. The product now sold as Hinder® was originally developed in the 1950s by Leffingwell Chemical Co. as a spreader-sticker for use on citrus. Its value as a deer repellent was noted by citrus growers. Field tests to evaluate “Spreader Sticker 268” were conducted by Leffingwell in the late 1960s and early 1970s. It was first registered in California as a repellent in 1969 under the trade name “Repel.” It received Special Local Needs registration in many states under the trade name “Hinder®” and full federal registration in 1981. Ammonium soaps of higher fatty acids, as a concentrate, is a brown liquid with an odor of ammonia. It is soluble in water. Bye Deer® contains 85% active ingredient, and is labeled for use to “protect established and new plantings of shrubs, small trees, and flowers from damage caused by white-tailed deer.” Bye Deer® received federal registration as a deer repellent in April 1993. G-40 Toxicity For ammonium soaps of higher fatty acids, the acute oral LD50 for rats is > 5 g/kg. For rabbits, the acute dermal LD50 is > 2 g/kg.a aHinder® Technical Data Sheet, Agricultural Chemicals, UNIROYAL Inc. For Additional Information Fargione, M. J., and M. E. Richmond. 1992. The effectiveness of soap in preventing deer browsing. Proc. Eastern Wildl. Damage Control Conf. 5:68-74. FENTHION Chemical Name 0,0-dimethyl 0-[3-methyl-4-(methylthio)phenyl] phosphorothioate Trade Names Rid-A-Bird® 1100, Baytex, Queletox, Tiguvon, and others Use Fenthion is used in Rid-A-Bird® perches as a contact toxicant for birds (starlings, house sparrows, and pigeons). It has also been used as an insecticide. Species Acute Oral LD50 (mg/kg) MAMMALS Mouse Rat Rabbit 88a a 180 - 615b 150a History BIRDS The compound was developed by G. Schrader and E. Schegk and introduced in 1957 as an experimental insecticide by Farbenfabriken Bayer. It has been used to control Quelea quelea, a weaver bird, in Africa by spray applications to roosts and nesting colonies. Canada goose 12.0c Duck 5.9a American kestrel 1.0 - 1.33d Bobwhite < 4.0c Quail, Coturnux 17.8b,d Quail, California 15.0c Pheasant 17.8c Chicken 15 - 28b Pigeon 1.78d - 4.63a,c Mourning dove 2.37d - 2.5c Black-billed magpie 4.22 - 5.62d American robin 5.62d Starling 5.30d - 17.8b House sparrow 5.62b - 22.7c Red-winged blackbird 1.78b - 3.50d Common grackle 4.21b - 7.50d House finch ~10c - 13.3d Properties In pure form, it is a colorless liquid. The technical material is a yellow to brown oily liquid with a weak garlic odor. It is soluble in water and in most organic solvents. Pharmacology Fenthion is an organophosphate compound; the toxic action of these compounds is caused by their inhibition of acetylcholinesterase. Fenthion is readily absorbed through the skin. Death can occur within minutes but normally takes from 2 to 12 hours, depending on the level and mode of exposure. Toxicity Fenthion is moderately toxic to mammals but highly toxic to birds. The symptoms of intoxication include lacrimation, salivation, congestion, ataxia, immobility, toxic or chronic convulsions, and death. The toxicity is as follows: For Additional Information Jackson, W. B. 1978. Rid-A-Bird® perches to control bird damage. Proc. Vertebr. Pest Conf. 8:47-50. Schafer, E. W., Jr., W. A. Bowles, Jr., and J. Hurlbut. 1983. The acute oral toxicity, repellency and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environm. Contam. Toxicol. 12:355-382. Acute dermal LD50 values are reported as follows: mouse, 500 mg/kg; rat, 330 mg/kg; duck, 44 mg/kg.a The potential for secondary hazards associated with the use of fenthiontreated perches was documented for avian predators and scavengers in 1969. Because of the low mammalian toxicity, the potential hazard for mammalian scavengers or predators is much less. Secondary poisoning of raptors and owls following exterior use of perches in the winter has been reported. aSweet (1993) bHone and Mulligan (1982) cHudson et al. (1984) dSchafer et al. (1983) For complete citations, see References at the end of this section. G-41 GAS CARTRIDGES Chemical Components Variable, depending on type of gas cartridge. Trade Names US Department of Agriculture Gas Cartridge, Giant Destroyer®, Smoke’Em®, Gopher Gasser®, Dexol Gasser®, and others. Use Gas cartridges are incendiary devices designed to give off carbon monoxide and other poisonous gases and smoke when ignited. They are used to fumigate burrows of certain rodents and other mammals. History Gas cartridges were developed by the former Bureau of Biological Survey more than 30 years ago. One type is manufactured and supplied by the Pocatello Supply Depot, USDAAPHIS-Animal Damage Control, Pocatello, Idaho. Other types were developed and are manufactured and sold by private commercial establishments. Properties The current USDA gas cartridge was developed for control of woodchucks, ground squirrels, prairie dogs, and pocket gophers. It contains sodium nitrate, charcoal, and inert ingredients. A similar cartridge was developed and registered by USDA for fumigating coyote dens. G-42 Most gas cartridges are made of cardboard or paper and are ignited with a fuse. Care should be taken to avoid fire hazards at locations of use. Dry grasses, and methane or natural gas, which may be present in or around structures, can make the use of gas cartridges a potential fire hazard. Toxicity Two hundred parts per million of carbon monoxide in inhaled air may produce symptoms of poisoning in a few hours, and 1,000 ppm can cause unconsciousness in 1 hour and death in 4 hours.a aClark (1986) Pharmacology Gas cartridges give off smoke and toxic gases when ignited. Carbon monoxide gas is a major product. In humans, the first stage of carbon monoxide poisoning produces a feeling of tightness across the forehead, headache, throbbing at the temples, dizziness, weariness, nausea, vomiting, collapse, and unconsciousness. In the second stage, the blood pressure falls, muscular control is lost, intermittent convulsions may occur, and the victim’s breathing becomes shallower, slower, and finally stops. Presumably, carbon monoxide acts similarly on other animals. For complete citations, see References at the end of this section. For Additional Information Dolbeer, R. A., G. E. Bernhardt, T. W. Seamans, and P. P. Woronecki. 1991. Efficacy of two gas cartridge formulations in killing woodchucks in burrows. Wildl. Soc. Bull. 19:200-204. Matschke, G. H., and K. A. Fagerstone. 1984. Efficacy of a two-ingredient fumigant on Richardson’s ground squirrels. Proc. Vertebr. Pest Conf. 11:17-19. Savarie, P. J., J. R. Tigner, D. J. Elias, and D. J. Hayes. 1980. Development of a simple twoingredient pyrotechnic fumigant. Proc. Vertebr. Pest Conf. 9:215-221. METHYL ANTHRANILATE Chemical Names Methyl anthranilate; 0-aminobenzoic acid methyl ester; 0-carbomethoxyaniline Trade Name ReJeX-iT® Use Because methyl anthranilate is broadly (if not universally) repellent to birds, it has many potential applications. The development of several of these applications has begun, and the formal registration of a few is imminent. The manufacturer (PMC Specialties Group) anticipates the registration of methyl anthranilate as a bird repellent additive to standing water at airports. The company also anticipates registration of methyl anthranilate as a bird repellent additive to Concover® (Newastecon, Inc.), a product designed as a thin cover for landfill operations. Gulls and crows refuse to forage in areas sprayed with Concover®/methyl anthranilate. The next anticipated use for the compound is application to turf and cover crops as a goose repellent; this registration is expected in 1994. History Methyl anthranilate is a GRAS (generally recognized as safe) food flavoring that is approved by the Food and Drug Administration as an additive to both human and livestock feeds. This chemical occurs naturally and is the characteristic odor of Concord grapes. The major US producer is PMC Specialties Group. The company synthesizes the chemical as a precursor ingredient for the manufacture of calcium and sodium saccharin. The first publication on the bird repellency of methyl anthranilate appeared in Poultry Science (Kare and Pick 1960). The following year, methyl anthranilate was patented as a bird repellent. For reasons still not completely understood, methyl anthranilate is a chemical irritant to birds, much as ammonia, formaldehyde, and black pepper are irritants to mammals. Every avian species tested to date, including laughing gulls, ring-billed gulls, starlings, sparrows, waxwings, red-winged blackbirds, grackles, cowbirds, mallards, Canada geese, snow geese, crows, chickens, guinea fowl, pheasants, bobwhite quail, and turkeys will avoid normally preferred foods when these foods are adulterated with methyl anthranilate at concentrations ranging from 0.5% to 1.0% by weight. Pharmacology Properties aSweet (1993) Methyl anthranilate at room temperature is an oily yellowish liquid. It has a fruity or grapelike odor and occurs in neoli, ylang-ylang, bergamont, jasmine, other essential oils, and in grape juice. It can be obtained synthetically by esterifying anthranilic acid with CH3OH in the presence of HCl. Methyl anthranilate is only slightly soluble in water but is freely soluble in alcohol or ether. It has a boiling point of 256oC, a melting point of 24oC, and a specific gravity of 1.168. It has a vapor pressure of 1 mm at 20oC. According to the Materials Data Safety Sheet, the pure substance may be harmful if inhaled, ingested, or absorbed through the skin. The vapor or mist from the concentrated compound can be irritating to the eyes, mucous membranes, and upper respiratory tract. It can cause skin irritation. Toxicity Methyl anthranilate is not fundamentally toxic to mammals or birds. It may, however, be moderately toxic to fish. The acute oral LD50 for this compound is reported as follows: mouse, 3,900 mg/kg; rat, 2,910 mg/kg; guinea pig, 2,780 mg/kg. For dimethyl anthranilate, the reported LDL0 for the rat is 3,380 mg/kg.a For Additional Information Cummings, J. L., D. L. Otis, and J. E. Davis, Jr. 1992. Dimethyl and methyl anthranilate and methiocarb deter feeding in captive Canada geese and mallards. J. Wildl. Manage. 56:349355. Kare, M. R., and H. L. Pick. 1960. The influence of the sense of taste on feed and fluid consumption. Poultry Sci. 39:697-706. Mason, J. R., M. L. Avery, J. F. Glahn, D. L. Otis, R. E. Matteson, and C. O. Nelms. 1992. Evaluation of methyl anthranilate and starch-plated dimethyl anthranilate as bird repellent feed additives. J. Wildl. Mange. 55:182-187. G-43 METHYL BROMIDE Chemical Name Methyl bromide Trade Name Brom-o-gas® Use Pharmacology Methyl bromide is used as a fumigant for rats and mice, insects, certain soil pests, and for treating agricultural commodities. It is sometimes formulated to include a small amount of chloropicrin as a warning agent. Methyl bromide is harmful by inhalation of the vapors; injury may occur by eye or skin contact with the liquid. History Methyl bromide was first described by Perkin in 1884. Its early use was medicinal. It was later used in the preparation of methyl compounds employed in the manufacture of aniline dyes and still later as a fire extinguishing agent. Methyl bromide was first used in California as an insecticide in 1935 by D. B. Mackie, then chief of the Bureau of Entomology and Plant Quarantine, California State Department of Agriculture. In 1938, C. E. Berry reported success in killing ground squirrels in California by injecting methyl bromide into their burrow systems at the rate of 10 cm3 per burrow opening. In the same year, it was reported that all life stages of fleas in the rodent burrows could be killed at this same dosage. This was important from the standpoint of plague control. Properties Methyl bromide is a colorless liquid which boils at 4.5oC. At ordinary temperatures it is a nonflammable gas, 1 pound of which occupies 3.98 ft3 (0.25 m3/kg). Methyl bromide is 3.5 times heavier than air. It has a burning taste and slight chloroform-like smell. Some formulations contain a small amount of chloropicrin as a warning agent. G-44 In acute exposures by inhalation, the effects are on both the respiratory and central nervous systems. These effects may be somewhat delayed, but rarely for longer than 24 hours in the case of respiratory symptoms, and seldom over 48 hours for symptoms in the central nervous system. The delay in onset of symptoms of intoxication makes toxic exposure to methyl bromide possible before the hazard is appreciated. Repeated exposures to low concentrations will seldom have pulmonary effects, but central nervous system manifestations may occur days or weeks after the initial exposure. Methyl bromide will act as a lung irritant in the respiratory system. Effects may vary from mild bronchitis to pulmonary edema and respiratory failure. Signs and symptoms may include coughing, chest pain, difficult or painful breathing, and eventually “wet” breathing often complicated by bronchopneumonia. Central nervous system symptoms usually accompany or follow the respiratory effects by several hours. Signs and symptoms include intense nausea and vomiting, dizziness, blurred vision, staggering gait, and slurred speech. Convulsions are an ominous sign. Following excitation, central nervous system depression may occur. Muscle weakness and respiratory paralysis may also occur. Exposure to methyl bromide may produce permanent neurological disburbances such as muscular pain, diplodia, dizziness, or even mental deterioration. Persons surviving for more than 48 hours may experience nausea and vomiting for as long as a week and permanent neurological damage. Since methyl bromide has a very low boiling point, evaporation takes place very rapidly and within seconds it can entirely disappear from the surface of the skin. However, if methyl bromide is spilled on clothing, gloves, or shoes, such coverings may become saturated, and contact with the skin is prolonged. No particular sensation is produced by such skin contact and the individual may be unaware that exposure has occurred. As a result, burns similar to thermal burns may occur. Leather goods such as shoes that become contaminated with methyl bromide should be destroyed. No person should be permitted to handle methyl bromide while wearing gloves or close fitting clothes. Methyl bromide will penetrate ordinary rubber gloves, so these should not be worn. Where methyl bromide has been spilled on clothing, such clothing should be removed and carefully aerated before being reworn. Severe corneal burns may result from splashes of the liquid material in the eye. Vapor exposures to the eye will cause irritation, but burns are unlikely. Toxicity For humans, the reported inhalation LCL0 is 60,000 ppm for 2 hours, and the reported TCL0 is 35 ppm.a Single exposures of 1,000 ppm for 30 to 60 minutes are dangerous to life. Repeated exposures of 100 ppm for 7 hours per day can produce serious poisoning.b Humans should wear appropriate respirators whenever the concentration in the air exceeds 17 ppm.c For Additional Information Lewis, R. L. Sr., and D. V. Sweet. 1985. Registry of toxic effects of chemical substances. 198384 Supplement. US Dep. Health Human Serv., Centers for Dis. Control, Nat. Inst. Occupational Safety Health. Cincinnatti, OH. For rats, the reported LC50 is 302 ppm for 8 hoursa, and the reported LCL0 is 3,120 ppm for 15 minutesd. Methyl bromide is phytotoxic. Plants with roots in the vicinity of fumigated rodent burrows may be injured or killed. aSweet (1993) bClark (1986) cSpencer (1981) dLewis and Sweet (1985) For complete citations, see References at the end of this section. G-45 NAPHTHALENE Chemical Name Naphthalene Use An insecticidal fumigant of limited usefulness and somewhat low potency. Because of its pungent odor, it is sometimes used as a mammal repellent, in paticular for repelling unwanted animals from beneath houses or from attics. It is currently registered for rabbits, squirrels, bats, dogs, and birds and is one of two active ingredients in a registered snake repellent. History Naphthalene has long been used as a fumigant and repellent for the clothes moth. Properties Colorless, flaky crystals with a melting point of 80oC. Insoluble in water; slightly soluble in alcohol; soluble in ether, chloroform, and other organic solvents. G-46 Pharmacology Prolonged inhalation may cause headache, nausea, vomiting, and sweating, followed by anemia, haematuria, and optic neuritis. Skin contact may cause local irritation and occasionally dermatitis. In accidental ingestions, naphthalene is rapidly absorbed from the gastrointestinal tract of children. Ingestion causes abdominal pain, diarrhea, rapid pulse and respiration, fever and rigors, and excitement leading to unconsciousness, coma, and convulsions. Some individuals, particularly males with a Mediterranean or African ancestry, may have a severe hemolytic crisis which may be delayed for several days after exposure. Dermal absorption of naphthalene from blankets has been reported to have caused deaths in extremely young Greek infants. Toxicity The acute oral LD50 of naphthalene for mice is reported to be 533 mg/kg; for rats, 490 mg/kg. The acute oral LDL0 for cats is reported as 100 g/kg; for dogs, 400 mg/kg; and for a human child, 100 mg/kg.a aSweet (1993) For complete citations, see References at the end of this section. RED SQUILL Other Names Squill, scilliroside glycosides (active ingredients) Use Red squill powders and extracts have been used primarily for the control of Norway rats. Red squill can be used successfully with all food baits that can be finely ground and mixed thoroughly. It is not satisfactory in cubed fruits or vegetables because these materials will not take up enough squill to kill rats. History Red squill, often referred to as the sea onion, is a perennial onionlike plant (Urginea maritima), belonging to the lily family and native to countries around the Mediterranean Sea. The bulbs, weighing 5 to 6 pounds each, are sliced, dried, and ground to a fine reddish powder. Red squill has been used since ancient times in the Mediterranean area to combat rats. Until 1945 it was difficult to obtain red squill powders of uniform toxicity. Then a method of fortification was developed whereby “weak” squill powders could be made more toxic and still retain the emetic qualities possessed by the original powder. Properties Red squill has been available in powdered form, as a liquid extract, and in a paste. The powder is hygroscopic—it absorbs water from the atmosphere and cakes and hardens if exposed to the air. The powder is extremely irritating to the skin, only slightly soluble, has low toxicity, is not cumulative, is not absorbed by the skin, and is relatively safe to humans and nontarget animals that can vomit such as dogs, pigs, and sometimes cats. Its most desirable quality is this natural emetic aciton which causes vomiting in most animals. Since rats are unable to vomit, they are unable to get rid of the bait through emesis. Red squill has a sharp, unpleasant, bitter taste but is fairly well accepted by Norway rats initially, if not used in proportions greater than 10% of the finished bait material. Most species of domestic animals will reject baits containing red squill. Its bitter taste can be a major disadvantage, however, because it may cause bait shyness in rats consuming a sublethal dose in the initial feeding. Also, red squill bulbs, powders, or extracts deteriorate in ratkilling ability when subjected to temperatures over 80oC. The powder slowly loses toxicity when it remains in contact with the air and retains only one-half of its original potency when stored for 4 years under ordinary warehouse conditions. Toxicity is preserved when the material is kept in hermetically sealed containers. Pharmacology The toxic action of red squill depends on the presence of a rodent-toxic glycoside, scilliroside, in the plant. It kills by a digitalis-like action that causes heart paralysis. Most rats die within 12 hours of eating a lethal dose. Toxicity Male rats are considerably more resistant to squill than are females. The toxicity of red squill has been reported as follows: Species Mouse Norway rat (female) Norway rat (male) Cat Dog Pig Goat Cattle (juvenile) Cattle (adult) Acute Oral LD50 (mg/kg) 50a 200 - 250b 490c 100c 145c 200c 500c 100c 250c Red squill is considered essentially nontoxic to poultry. aSweet (1993) bBartik and Piskac (1981) cClarke et al. (1981) For Additional Information Bartik, M., and A. Piskac, eds. 1981. Veterinary toxicology. Elsevier Sci. Pub. Co., Amsterdam, Oxford, and New York. 346 pp. Clarke, M. L., D. C. Harvey, and D. J. Humphreys. 1981. Veterinary toxicolgy. 2nd ed. Bailliere Tindall, London. 328 pp. Verbiscar, A. J., T. F. Banigan, and H. S. Gentry. 1986. Recent research on red squill as a rodenticide. Proc. Vertebr. Pest Conf. 12:5156. G-47 SODIUM CYANIDE Chemical Name Sodium cyanide Use Properties Sodium cyanide has been used as a fumigant to kill insects or mammals in burrows. Currently it is used in the M-44® device for control of coyotes, foxes, and feral dogs that depredate livestock and poultry or that depredate federally designated threatened or endangered species. One product is also registered for use to control vectors of communicable diseases (such as canids that may be carrying rabies). Sodium cyanide is a colorless solid with a melting point of 564oC. It readily absorbs moisture from the air and is very water soluble in water. When in contact with carbon dioxide or acids, it forms hydrogen cyanide (HCN) gas, which is extremely toxic. History Sodium cyanide has been used in predator control in the United States since the invention of the Coyote Getter in the 1930s. This device, a precursor of the current M-44® device, utilized a dose of sodium cyanide propelled into the mouth of a coyote by a .38-caliber shell containing a primer. All predacidal uses of sodium cyanide were cancelled by the EPA in March 1972. Sodium cyanide was re-registered for use in the M-44® device in 1975. G-48 Pharmacology Hydrocyanic acid (HCN) is highly and quickly toxic by contact, ingestion, or inhalation of vapors. It poisons the cytochrome-oxidase system of all cells. Unconsciousness occurs rapidly, followed by convulsions and death within 5 minutes. Extreme caution must be used. Toxicity The acute oral LD50 of sodium cyanide for rats is 6.44 mg/kg. The reported human acute oral LDL0 is variously reported as 2.86 mg/kg or 6.56 mg/kg.a The acute oral human TDL0 is reported to be 0.7 mg/kg.a For HCN, the threshold limit value/time weighted average is 10 ppm for humans.b It is immediately dangerous to life or health at 50 ppm.c A concentration of 200 ppm will quickly kill a human.b An antidote kit containing amyl nitrate is available and effective if used quickly following exposure. aSweet (1993) bSpencer (1981) cBerg (1983) For complete citations, see References at the end of this section. For Additional Information Connolly, G. 1988. M-44 sodium cyanide ejectors in the animal damage control program, 1976-1986. Proc. Vertebr. Pest Conf. 13:220-225. Shult, M. J., C. W. Ramsey, and W. G. Klussmann. 1976. Using the M-44 in coyote control. Texas Agric. Ext. Serv. Pub. MP1181, College Station, Texas. SODIUM FLUOROACETATE Chemical Name Sodium monofluoroacetate Other Names Compound 1080®, 1080, Fluoroacetate Use Compound 1080 is a single-dose toxicant currently registered for use in the Livestock Protection Collar for control of coyotes that are depredating sheep or goats in fenced pastures only. It was formerly registered for use against commensal rodents and certain field rodents in some states. It was also formerly used in large meat draw stations for the control of coyotes and other canids. History Polish scientists discovered that an organic fluorine compound from “ratsbane” (Dichapetalum toxicarium), a West African poisonous plant, had possibilities as an economic poison. The information was disclosed in 1942 to British scientists who sent samples of the compound to the United States. It received the designation “1080” from the invoice number given to it at the Patuxent Wildlife Research Center, where it was first tested as a rodenticide in June 1944. When the compound appeared promising, the remainder was forwarded to the (then) US Fish and Wildlife Service Denver Wildlife Research Center for further testing and development. More recently, the compound was researched for use in the Livestock Protection Collar, a 1080filled rubber bladder placed around the neck of a goat or sheep, designed to kill an attacking coyote. Properties Sodium monofluoroacetate is a white, stable, water soluble, practically tasteless, crystalline compound. It has a faint acetate odor and a mild acid-salty taste. When the powdered material is exposed to air, it takes up water and becomes gummy. It decomposes at approximately 200oC and is unstable above 110oC. The material is extremely soluble in water and relatively insoluble in many organic solvents and in vegetable fats and oils. A black dye has often been added to the compound during manufacturing to distinguish it from substances like sugar and flour. Degradation. Salts of monofluoroacetic acid are readily absorbed by root tissues and other cellulosic materials, and are decomposed adaptively by soil bacteria, apparently of the genus Pseudomonas. Therefore, any sodium monofluoroacetate leached into the soil will likely be held in the upper layers rich in microorganisms, and decomposed by bacteria. In tests conducted in England the compound either exhibited no measurable toxicity from the start or exhibited no measurable toxicity within two weeks, depending upon the soil type, when applied to soils at 10 ppm; it exhibited no measurable toxicity within 11 weeks when applied to soils at 50 ppm. Translocation. Sodium monofluoroacetate leached into the soil may be taken up by plants. However, only a small amount is translocated upward to the leaves; the rest remains adsorbed in the roots. Plants can decompose the compound. In one experiment plants had decomposed 29% of the absorbed sodium monofluoroacetate after 48 hours of incubation. Pharmacology Sodium monofluoroacetate is very rapidly absorbed in the gastrointestinal tract. Symptoms of poisoning may appear in 15 to 45 minutes and death may occur some later time but usually within 24 hours. The compound is not accumulative to any practical degree. Demonstration of symptoms varies widely among species. In the body, sodium monofluoroacetate, like other monofluoroacetates, is metabolized to highly toxic fluorocitrate. Fluorocitrate blocks the Krebs cycle, the major mechanism for releasing energy from food. The resulting buildup of citrate blocks glucose metabolism, another mechanism for releasing energy from food. The blockage of these processes causes the energy supply to be reduced to a point where cellular permeability barriers are destroyed, resulting in a loss of function and finally cellular death. Eventually, gross organ or organ system disorders are manifested. Death results from cardiac and/or central nervous system failure. The primary pharmacological action of Compound 1080 may be either in the central nervous system or the heart — or a combination of the two — depending on the subject involved. Central nervous system symptoms are characterized by initial intermittent convulsions followed by depression. The heart symptoms appear later, and in primates, heart action terminates in ventricular fibrillation. There is no practical antidote for 1080 poisoning; treatment is symptomatic. Once fibrillation begins, death is assured. Compound 1080 is slower in its action than strychnine. A dog poisoned with 1080 becomes extremely excited, frequently howls, and exhibits running fits, nonrecognition of human presence, and action suggestive of fearful hallucinations or hysteria. The terminal phase of 1080 poisoning in a dog G-49 involves a tonic spasm (continuous muscular contractions) followed by running movements executed in a prone position. The course of tonic spasms may subside at times and the dog may appear normal, but ultimately, repeated convulsive anoxic assaults on the respiratory center lead to death through respiratory paralysis. Death is seldom attributable primarily to action on the heart. Secondary poisoning can be demonstrated under some conditions with sodium monofluoroacetate. However, simple dilution and the fact that animals can metabolize 1080 to nontoxic metabolites and/or excrete a large quantity of a dose prior to death considerably reduces the hazard of acute poisoning via secondary sources. Toxicity An outstanding characteristic of the toxicity of Compound 1080 is the extremely wide variation in susceptibility between different species of animals. Dogs have been killed with a dosage of 1080 as small as 0.06 mg/kg, but a Norway rat may require 8.0 mg/ kg. Species vary considerably in their response, with primates and birds the least sensitive, and carnivores and rodents generally the most susceptible. The amount necessary to produce lethal effects often varies in different strains of the same species. The LD50 is 2.5 to 8 mg/kg for Rattus norvegicus and 1 to 4 mg/kg for Rattus rattus.a In general, birds are more tolerant of 1080 than are most mammals, although there are exceptions. Dogs and coyotes are particularly sensitive to Compound 1080. Dogs, with an LD50 of 0.06 mg/kg, and coyotes at 0.12 mg/kg, can be poisoned secondarily by eating 1080-killed rodents. Chickens and birds in general are not very susceptible to poisoning from Compound 1080, having an LD50 in the range of 5 to 10 mg/kg. Compound 1080 is not hazardous to fish; tests have shown fingerling bream and bass to live indefinitely without discomfort in a concentration of 370 ppm. Judging from fatal and near-fatal cases, the dangerous dose to a human is 0.7 to 2.1 mg/kg.a G-50 Toxicity Table Acute Oral LD50 (mg/kg)b Species MAMMALS Carnivores Bear, Ursus sp. Domestic cat Coyote, Canis latrans Dingo, Canis familiaris dingo Dog, Canis familiaris Desert kit fox, Vulpes macrotis Domestic ferret, Mustela putorious Rodents Ground squirrels Fisher’s, Spermophilus beecheyi fisheri Pocket gophers: Northern, Thomomys talpoides Rats Norway (lab), Rattus norvegicus (male) Rattus norvegicus (female) Norway (wild), Rattus norvegicus Alexandrine, Rattus rattus alexandricus Black, Rattus rattus sp. Cotton, Sigmodon hispidus litteralis Woodrat, Neotoma intermedia Mice Deer mouse, Peromyscus sp. House mouse, Mus musculus Miscellaneous spp. Meadow vole, Microtus pennsylvanicus Prairie dog, Cynomys ludovicianus Lagomorphs Black-tailed jackrabbit, Lepus californicus European rabbit, Oryctolagus cuniculus 0.5 - 1.0 0.4 - 0.5d 0.12e 0.11d 0.05 - 1.0d 0.22d 1.41 0.3 0.33c 2.1* 2.2* 3.0 0.5 0.1 0.1 1.5 4.0 4.0 - 17.0d 0.92 0.3 5.55 < 0.8d *Research has shown much variation between strains of laboratory rodents. aClark (1986) bValues as reported by Atzert (1971) except as otherwise noted cDenver Wildlife Research Center, USDA, files dHone and Mulligan (1982) eConnolly (1980) For complete citations, see References at the end of this section. Precautions First Aid Treatment for Dogs Sodium monofluoroacetate should be used by trained personnel only, and then under conditions that afford maximum security against accidental poisoning. Users of the Livestock Protection Collar are required to complete thorough training prior to certification. The 1080 solution may escape from collars that are punctured or otherwise damaged. Protective safety measures should always be taken when 1080 solutions are handled. Lloyd (1983) notes that in practically all cases, treatment of poisoned dogs is ineffective, and the prognosis of poisoned animals is grave. If a practitioner has the opportunity to treat an affected dog, the best that can be hoped for is palliation or symptomatic relief. Intravenous injections of barbiturates q.s. to control convulsions have been used. Injections of calcium gluconate solutions have also been employed to control tetany, which is thought to be caused by a lactic acidemia. Attempts to decrease the concentration of citrate have involved the administration of acetate. Monoacetin (glyceryl monoacetate) has been given intramuscularly in doses of 0.55 gm/kg. Additionally, solutions containing 50% alcohol and 5% acetic acid have been given orally in doses of 8.8 ml/kg (Lloyd 1983). For Additional Information Atzert, S. P. 1971. A review of sodium monofluoroacetate (Compound 1080) — its properties, toxicology, and use in predator and rodent control. Bur. Sport Fish. Wildl., Div. Wildl. Serv., Spec. Sci. Rep.-Wildl. No. 146, US Dep. Inter., Washington, DC. 34 pp. Calver, M. C., and D. R. King. 1986. Controlling vertebrate pests with fluoroacetate: lessons in wildlife management, bio-ethics, and coevolution. J. Biol. Educ. 20(4):257-262. Connolly, G. E. 1980. Use of Compound 1080 in livestock neck collars to kill depredating coyotes; a report on field and laboratory research, November 1978 — March 1980. US Dep. Inter., Fish Wildl. Serv., Denver, Colorado. 125 pp and append. Connolly, G., and R. J. Burns. 1990. Efficacy of Compound 1080 livestock protection collars for killing coyotes that attack sheep. Proc. Vertebr. Pest Conf. 14:269-276. Kun, E. 1982. Monofluoroacetic acid (Compound 1080), its pharmacology and toxicology. Proc. Vertebr. Pest Conf. 10:34-41. Lloyd, W. E. 1983. Sodium fluoroacetate (Compound 1080) poisoning. Pages 112-114 in R. W. Kirk, ed. Current veterinary therapy VIII: small animal practice. W. B. Saunders Co., Philadelphia. Rammell, C. G., and P. A. Fleming. 1978. Compound 1080. An. Health Div. Minist. Agric. Fisheries, Wellington, New Zealand. 112 pp. Wade, D. A. 1977. Standard guideline for the use and development of sodium monofluoroacetate (Compound 1080) as a predacide (ASTM designation E 590- 76). Pages 157-170 in W. B. Jackson and R. E. Marsh, eds., Test methods for vertebrate pest control and management materials. ASTM STP 625, Am. Soc. Testing and Mater. Philadelphia. Howard, W. E., and R. H. Schmidt. 1984. Biological rationale for 1080 as a predacide. Proc. Vertebr. Pest Conf. 11:138-145. G-51 STARLICIDE® Chemical Name 3-chloro-p-toluidine hydrochloride Other Names 3-chloro-4-methyl benzylnamine hydrochloride, CPTH, DRC-1339 Use Starlicide® is a slow-acting avicide registered for the control of starlings, blackbirds, pigeons, gulls, ravens, crows, and magpies. History This chemical, originally coded DRC1339 and evaluated by the Denver Wildlife Research Center, was found to be an excellent toxicant for starlings and blackbirds when formulated as a Starlicide® pellet. It received federal registration in 1967 for feedlot uses. Starlicide® is manufactured and distributed by the Purina Mills Company. Registration of a DRC-1339 concentrate has been maintained by USDAAPHIS for use against starlings, blackbirds, and gulls, with additional approvals granted for use against pigeons in 1992 and against ravens, crows, and magpies in 1993. Use of the DRC-1339 concentrate is restricted to USDA-APHIS personnel. Properties The technical compound is a pale yellow, crystalline solid material that is very soluble in water and other highly polar solvents; it sublimes at 220oC. If formulated with many grains, potency of the compound may decline significantly when stored. Commercial Starlicide® pellets retain their potency for 6 to 12 months. Pharmacology Starlicide® is a slow-acting and apparently painless toxicant in birds and mammals. In sensitive bird and mammal species, death results primarily from uremia (a buildup of uric acid in the blood). Death occurs without convulsions or spasms, and is the result of generalized circulatory impairment in the liver and kidney, and congestion of the major organs. At death, victims’ feathers are usually fluffed and their feet tucked inside the feathers of the lower breast. In most mammals and nonsensitive birds, death results from methemoglobinenemia (a buildup of methemoglobin in the blood). Mammals become listless and comatose before death. Birds and mammals appear to metabolize or excrete Starlicide® completely within a matter of hours, and the metabolites are also excreted. Known metabolites are nontoxic to birds and mammals. Because the Starlicide® and its metabolites are excreted while birds are still alive, there is no secondary toxicity to any scavengers eating dead birds. Toxicity In birds, the average time between ingestion and death is 36 to 60 hours, depending on the amount ingested. Even when the lethal dose level is exceeded many times, death still takes many hours. In most mammals, death occurs in 3 to 12 hours. The toxicity of Starlicide® varies considerably between bird species. Starlings, blackbirds, and crows are among the most sensitive birds; house sparrows and hawks are nonsensitive. Mammals are generally not sensitive to the toxic effects of Starlicide®. Toxicity Tables Species Acute oral LD50 (mg/kg)a,d BIRDS Mallard Blue-winged teal Pintail Cooper’s hawk Golden eagle Marsh hawk Kestrel Ring-necked pheasant Coturnix quail Domestic turkey Domestic chicken White-winged dove Mourning dove Pigeon (rock dove) Barn owl Blue jay Scrub jay Black-billed magpie Common raven Common crow American robin Starling House sparrow Red-winged blackbird Tricolored blackbird Boat-tailed grackle Common grackle Cassin’s finch House finch White-crowned sparrow Species 17.8 31.6 > 32 562 > 100 100 > 320 10 2.24 - 10 5.62 4b 4.22 3.16 - 7.50 17.8 4.22 10 1.78 5.6 - 17.7 5.62 1.33 - 1.78 3.16 3.16 - 4.11 320 - 448 2.41 2.74 1.00 1.00 > 100 > 225 > 320 Acute oral LD50 (mg/kg)c MAMMALS Mouse White mouse White rat Rat Dog Sheep Cow 2,000 960 1,170 - 1,770 655b > 100 ca 400 > 10 aDeCino et al. (1966) bSweet (1993) cClark (1986) dSchafer et al. (1983) For complete citations, see References at the end of this section. G-52 For Additional Information Besser, J. F., W. C. Royall, Jr., and J. W. DeGrazio. 1967. Baiting starlings with DRC1339 at a cattle feedlot. J. Wildl. Manage. 31:48-51. DeCino, T. J., D. J. Cunningham, and E. W. Schafer. 1966. Toxicity of DRC- 1339 to starlings. J. Wildl. Manage. 30:249-253. Knittle, C. E., J. L. Guarino, P. C. Nelson, R. W. DeHaven, and D. J. Twedt. 1980. Baiting blackbird and starling congregating areas in Kentucky and Tennessee. Proc. Vertebr. Pest Conf. 9:31-37. Knittle, C. E., E. W. Schafer, and K. A. Fagerstone. 1990. Status of compound DRC1339 registrations. Proc. Vertebr. Pest Conf. 14:311-313. Schafer, E. W., Jr., W. A. Bowles, Jr., and J. Hurlbut. 1983. The acute oral toxicity, repellency and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environ. Contam. Toxicol. 12:355-382. Sterner, R. T., D. J. Elias, and D. R. Cerven. 1992. The pesticide reregistration process: collections of human health hazards data for 3-chloro-p-toluidine hydrochloride (DRC1339). Proc. Vertebr. Pest Conf. 15:62-66. G-53 STRYCHNINE Chemical Name 2,4a,5,5a,7,8,15,15a,15b,15c-dechydro-4,6-methano-6H,14H-indolo (3,2,1-ij)oxepino(2,3,4-de)pyrrolo(2,3-h)quinolin-14-one Use Pharmacology Toxicity Strychnine is a widely used toxicant registered for use in controlling certain rodent and depredating bird species. In the past, strychnine was commonly used for controlling rodents, depredating birds, and mammals such as skunks and coyotes. Aboveground uses were halted by court action in 1988, but it remains registered and used belowground for control of pocket gophers and, in some states, other species. Strychnine acts the quickest of the commonly used rodenticides. It is not stored in body tissues nor absorbed through normal intact skin. It has a very slight odor, very high toxicity, and acts somewhat variably on target animals. Strychnine enters the blood very rapidly and acts on the central nervous system. The time of action depends on whether the stomach is empty or full and the nature of the food present. Animals with little in their stomachs react more quickly to strychnine than those that have fed recently. Symptoms may appear from 5 to 30 minutes after ingestion. LD50 values range between a low of 0.70, 0.75 and 1.5 mg/kg for coyotes, desert kit foxes, and black-tailed prairie dogs, respectively, to a high of 27.0 mg/kg for nutria; and between 16.0 mg/kg for chukar partridge and 24.7 mg/kg for ring-necked pheasants. LD50s for mallards, Canada geese, golden eagles, and house sparrows fall within an approximate range of 3.0 to 5.0 mg/kg. History Strychnine is one of the alkaloids processed from raw dried ripe seed of Strychnos nux vomica, a small tree native to India, North Australia, Vietnam, and Ceylon. This alkaloid was discovered by Pelletier and Caventon in 1817. There is 2.0% to 2.7% total alkaloid found in the seeds, which were used to kill dogs, cats, and birds in Europe at least as early as 1640. Properties Strychnine, a white crystalline powder, is currently available in an alkaloid form; the sulfate form previously used is no longer registered. Strychnine has a bitter taste. It is almost entirely insoluble in water and very stable; however, it is subject to acid-salt formation, which renders it water soluble and subject to leaching in acid soils. G-54 Intoxicated animals have frequent tetanic convulsions interspersed with quiescent periods. Ultimately these convulsions lead to death through respiratory failure. Strychnine is not assimilated into tissues or bone; however, residues in the gastrointestinal tract of animals poisoned with lethal doses are known to be potentially hazardous if the gastrointestinal tract is consumed. With its current belowground application pattern, secondary poisoning is unlikely. Livestock are about as sensitive to strychnine as rats.a Horses, hogs, geese, and ducks show no hesitation in eating strychnine baits. Cattle and sheep are more reluctant to accept baits. Gallinaceous game birds and most domestic poultry, however, are less susceptible to strychnine than most rodents. Antidote The use of general antidotes is feasible and often successful if treatment is initiated soon after exposure. Sodium pentobarbital and sodium amytal both act to reduce the severity of convulsions in humans (see J. Am. Med. Assoc. 100:548-551). Emetics such as 1% to 2% tannic acid are useful but should only be used after the convulsive stage is past. Prompt administration of methocarbamol is useful in treating poisoned dogs. Prognosis: if the patient lives for 24 hours, he or she will probably recover. Strychnine Alkaloid Acute Oral LD50 (mg/kg) MAMMALS Carnivores Cat Coyote Dog Desert kit fox Rodents Squirrels California ground squirrel Black-tailed prairie dog Pocket gophers Northern pocket gopher Rats & Mice Rat Norway rat (wild) White mouse (lab) White rat (male) White rat (female) Black rat Polynesian rat Miscellaneous Rodents Banner-tailed kangaroo rat California meadow mouse Meadow vole Nutria Lagomorphs Black-tailed jackrabbit Ungulates Cow Mule deer Horse Sheep Swine Swine Primates Human Human Human 0.5f - 2.0c 0.7b 0.5f - 1.2b 0.7b 19.9 - 28.0b 1.5b For Additional Information Osweiler, G. D. 1983. Strychnine poisoning. Pages 98-100 in R. W. Kirk, ed. Current veterinary therapy VIII: small animal practice, W. B. Saunders Co., Philadelphia. Palmateer, S. D. 1990. Registration status of vertebrate pesticides with emphasis on 1080 and strychnine. Proc. Vertebr. Pest Conf. 14:113-115. Schafer, E. W., Jr. 1984. Potential primary and secondary hazards of avicides. Proc. Vertebr. Pest Conf. 11:217-222. 8.3b 3 - 6e 12.0b 9.3b 14.0b 5.8b 10.1b 6.8b 3.7b 22.2b 6.8b 27.0 - 42.0b 4.4a 15.0b 17.0 - 24.0d 2.0b 7.5b 0.5 - 1.0c 10.0b 1c 15 - 30c 30 - 60e AMPHIBIANS Bullfrog 2.2d BIRDS Ducks and Geese Mallard Canada goose Raptors Golden eagle Galliformes Chicken Chukar Coturnix (Japanese quail) Pheasant California quail Pigeons and Doves Pigeon (rock dove) Passerines House finch Robin English sparrow 2.3d - 2.9c 4.0a 4.8 - 8.1d 5.0c 16.0d 22.6d 24.7d 112d 21.3d 5.6b > 10.0a 4.2d aClark (1986) bDenver Wildlife Research Center, USDA, files cHone and Mulligan (1982) dHudson et al. (1984) ePinto and Spear (1980) fSweet (1993) For complete citations, see References at the end of this section. G-55 THIRAM Chemical Name Tetramethylthiuram disulfide Other Names TMTD, Arasan Use Pharmacology Toxicity A fungicide which also has been used as a repellent to protect plants from deer, rabbits, rodents, and moles. The toxicity of thiram is as follows: Properties Thiram is of relatively low mammalian toxicity but is reported to cause skin irritation. As a repellent, it is presumed to work by taste, not odor. In some animals, a learned aversion may occur. For humans, ingestion may cause nausea, vomiting, diarrhea, and anorexia, followed by ataxia, hyperexcitability, and hypothermia. Hypotonia may progress to flaccid paralysis and respiratory failure. Skin contact or inhalation may cause irritation of the nose, throat, or skin and may induce an allergic dermatitis. A colorless crystalline material with a melting point of 155o to 156oC. Its solubility in water is 17.4 mg/l. It is slightly soluble in alcohol and in ether; it is soluble in chloroform and in acetone. A related compound, tetraethylthiuram disulfide (Antabuse) blocks the in vitro oxidation of ethanol at the acetaldehyde stage and is used for the treatment of chronic alcoholism. History Developed for fungicidal use in 1931 by E. I. duPont de Nemours and Company. The DuPont trade name, Arasan, is no longer used since other companies are now involved in manufacture and formulation of the fungicide. Persons handling or using thiram should avoid ingestion of alcohol before or after use. G-56 Species Acute Oral LD50 (mg/kg) Mouse Rat Rabbit Sheep Pheasant Poultry Mallard 1,350a 560 - 780a,b 210a 225c 673d ~1,000c >2,800d aSweet (1993) bBerg (1983) cHumphreys (1988) dHudson et al. (1984) For complete citations, see References at the end of this section. For Additional Information Radwan, M. A. 1969. TMTD wild animal repellents: review and current status. For. Sci. 15:439-445. ZINC PHOSPHIDE Chemical Name Zinc phosphide Use Zinc phosphide, at concentrations of 0.75% to 2.0% on grain, fruit, or vegetable baits, has been used successfully against such species as meadow mice, ground squirrels, prairie dogs, Norway rats, Polynesian rats, cotton rats, and nutria. In some areas, zinc phosphide baits have been partially or completely rejected by ground squirrels and meadow mice and at times control has been erratic. History Zinc phosphide appears to have been first synthesized by Marggral in 1740 and was first used as a rodenticide by the Italians in 1911. Extensive use of zinc phosphide in the United States did not occur until 1942, when the availability of strychnine became uncertain due to the war. Properties Zinc phosphide is a heavy, finely ground gray-black powder that is practically insoluble in water and alcohol. When exposed to moisture, it decomposes slowly and releases phosphine gas (PH3). Phosphine, which is highly flammable, may be generated rapidly if the material comes in contact with dilute acids. Zinc phosphide concentrate is a stable material when kept dry and hermetically sealed. Although zinc phosphide baits have a strong, pungent, phosphorous-like odor (garliclike), this characteristic seems to attract rodents, particularly rats, and apparently makes the bait unattractive to some other animals. For many uses of zinc phosphide formulated on grain or grain-based baits, prebaiting is recommended or necessary for achieving good bait acceptance. In general, zinc phosphide is less toxic than Compound 1080 or strychnine and is slower-acting than either of these compounds. There is only a small amount of deterioration of zinc phosphide on baits due to the evolution of phosphine gas; therefore, dry baits must be considered to be toxic indefinitely and must be used accordingly. Lecithin-mineral oil, added to zinc phosphide to adhere it to grain bait, offers protection against moisture, and therefore may increase its stability. Under field conditions, zinc phosphide baits may remain toxic for several months until baits are eroded by weathering, the carrier decomposes, or the grain is removed by insects. Physical erosion does not seem to occur rapidly. In one instance, zinc phosphide-treated bait exposed in the field for 2 to 3 months and subjected to 10 to 12 inches (25 to 30 cm) of rain continued to maintain some toxicity. When zinc phosphide is dusted onto wet baits, such as meats or cubed fresh fruits and vegetables, it breaks down within a few days and the baits soon lose their attractiveness. In soil, zinc phosphide breaks down rapidly to phosphine, which is either released into the atmosphere or converted to phosphates and zinc complexes. Translocation of phosphine gas has been demonstrated, but it is rapidly converted to harmless phosphates. There is no evidence that hazards exist via this route when grain baits are used in growing vegetables. Pharmacology When zinc phosphide comes into contact with dilute acids in the stomach, phosphine (PH3) is released. It is this substance that probably causes death. Animals that ingest lethal amounts of bait usually succumb overnight with terminal symptoms of convulsions, paralysis, coma, and death from asphyxia. If death is prolonged for several days, intoxication occurs that is similar to intoxication with yellow phosphorous, in which the liver is heavily damaged. The surface of the liver will be spotted and discolored. Prolonged exposure to phosphine can produce chronic phosphorous poisoning. Early symptoms of zinc phosphide poisoning are nausea, vomiting (yielding black stomach contents and the smell of phosphine), abdominal pain, chest tightness, excitement, and a feeling of coldness. In fatal cases, there is liver, kidney, and heart damage. The time between ingestion and death is frequently about 30 hours. Victims who are alive after 3 days are said to recover completely. Mild poisoning from breathing minute amounts of phosphine gas can be mistaken for food poisoning because of the diarrhea and stomach pains produced. Zinc phosphide-poisoned rats show no signs of distress until a short terminal death agony occurs. They typically die in a prone position with their legs and tails outstretched. Because zinc phosphide is not stored in muscle or other tissues of poisoned animals, there is no secondary poisoning with this rodenticide. The bait, however, remains toxic up to several days in the gut of a dead rodent. Other animals can be poisoned if they eat enough of the gut content of rodents recently killed with zinc phosphide. G-57 Toxicity Zinc phosphide is poisonous to some degree to all animals. Supposed safety factors such as the odor and dark color may be of little deterrence in some situations. As little as a teaspoonful of bait containing zinc phosphide could cause toxic symptoms in a child to whom the color and odor may not be disagreeable. Therefore, around dwellings, bait should be exposed only in situations that will prevent pets and children from coming into contact with it. Use extreme care in handling zinc phosphide concentrate and treated bait. If zinc phosphide baits are prepared in the open air, phosphide generated from the moist bait offers little hazard. When quantities of bait are prepared within a bait mixing plant, safeguards against continued exposure to low concentrations of phosphide must be taken. Zinc phosphide dust created by the preparation or handling of baits is also hazardous. Personnel working indoors should wear appropriate respirators and work under exhaust fans. Zinc phosphide baits should not be mixed or distributed with the bare hands. Oils, liquid or semisolid, are used in some preparations. Because phosphorous is soluble in certain fatty oils, it may be absorbed in small amounts through the skin. Continued exposure to phosphorous absorption may result in toxic manifestations at some later time. Rubber or synthetic gloves are preferrable when handling dry zinc phosphide bait formulations but cotton or leather gloves are acceptable. Zinc phosphide can be used for rat control on almost any food product; however, it (or any other acute toxicant) should not be used on bait materials recognizable as food in the home environment. Do not use on such foods as tomatoes, apples, oranges, or bread, unless they are made unrecognizable by rolling or cubing them. G-58 Toxicity Table 1.a,b Species Acute Oral LD50 (mg/kg) MAMMALS Carnivores Cat and dog Desert kit fox Rodents Squirrels California ground squirrel Black-tailed prairie dog Pocket gophers Northern pocket gopher Rats Norway rat (wild) White rat Black rat Roof rat Polynesian rat Mice Mouse Deer mouse Meadow vole California meadow mouse Other Rodents Banner-tailed kangaroo rat Muskrat Nutria Woodrat (LD100) Other Mammals Black-tailed jackrabbit Cow Human (estimated MLD*) Human, female (LDL0) Pig 20 - 40c 20 - 40c 93.0 33.1 18.0 6.8 27 - 40c 55.5 21.0 2.9 - 40.5 23.0 40d 40.5 18.0 15.7 8.0 29.9 5.55 25.0 8.25 50.0 40.0 80d BIRDS Ducks & Geese Mallard Snow goose White-fronted goose Galliformes Chicken California quail Partridge Pheasant Other Birds Mourning dove Red-winged blackbird *MLD is minimum lethal dose or LDL0 13.0 - 35.7c 8.8 7.5 20 - 40c 13.5 26.7 8.8 - 26.7 34.2 23.7 Toxicity Table 2.a,b For Additional Information Zinc Phosphide Acute Oral Toxicity (mg/kg) Hood, G. A. 1972. Zinc phosphide—a new look at an old rodenticide for field rodents. Proc. Vertebr. Pest Conf. 5:85-92. Species LDL0 LD50 LD100 Snow geese White-fronted geese Mallards Pheasant Quail Dove 5-10 0-5 5-10 0-10 5-10 10-20 8.75 7.5 13.0 8.8 13.5 34.25 5-10 10-15 10-20 10-15 10-20 10-50 Marsh, R. E. 1988. Relevant characteristics of zinc phosphide as a rodenticide. Proc. Great Plains Wildl. Damage Control Workshop 8:70-74. Tietjen, H. P. 1976. Zinc phosphide—a control agent for black-tailed prairie dogs. US Dep. Inter. Fish Wildl. Serv., Wildl. Leaflet 509. aClark (1986) unless otherwise noted bHood (1972) unless otherwise noted cHone and Mulligan (1982) dSweet (1993) For complete citations, see References at the end of this section. G-59 ZIRAM Chemical Name Zinc dimethyldithiocarbamate Other Name Zerlate Use Properties Toxicity Currently this compound is used as the active ingredient in one rabbit repellent. Ziram is a white, odorless powder with a melting point of 246oC. It is not soluble in water slightly soluble in alcohol and in ether; moderately soluble in acetone; and soluble in dilute alkali, chloroform, and in carbon disulfide. It is stable under ordinary conditions but is decomposed by acids. The acute oral LD50 for rats is reported to be as low as 267 mg/kg.a The acute oral LD50 to mice is 480 mg/kg, and to rabbits 400 mg/kg.a Dogs reportedly tolerated 25 mg/kg per day for one month but not for one year; they ingested 5 mg/kg per day for one year without ill effects.b History Ziram was originally developed as an accelerator in rubber vulcanization. It has been used as a fungicide since the early 1930s and later received use as a rodent, rabbit, and bird repellent. Pharmacology Ziram’s repellency is presumably due to taste or physiological discomfort following ingestion. Ziram may irritate the skin and mucous membranes. Persons handling or using ziram should avoid ingestion of alcohol before or after use. G-60 aSweet (1993) bSpencer (1981) For complete citations, see References at the end of this section. REFERENCES Berg, G. L., ed. 1983. Farm chemical handbook. Meister Pub. Co., Willoughby, Ohio. Clark, J. P. 1986. Vertebrate pest control handbook. Div. Plant Ind., California Dep. Food Agric. Sacramento. Hayes, W. J., Jr. 1982. Pesticides studied in man. Williams and Wilkins, Baltimore. 672 pp. Hone, J., and H. Mulligan. 1982. Vertebrate Pesticides. Sci. Bull. 89, Dep. Agric. New South Wales, Australia. 130 pp. Hudson, R. H., R. K. Tucker, and M. A. Haegele. 1984. Handbook of toxicity of pesticides to wildlife, 2d ed. US Dep. Inter. Fish Wildl. Serv., Resour. Publ. 153. Washington DC. 90 pp. Humphreys, D. J. 1988. Veterinary toxicology, 3d ed. Bailliere Tindall, London. 356 pp. Kidd, H., and D. R. Jones, eds. 1991. The agrochemicals handbook, 3d ed. The Royal Soc. Chem. Cambridge, England. Lewis, R. L. Sr., and D. V. Sweet. 1985. Registry of toxic effects of chemical substances. 198384 Supplement. US Dep. Health Human Serv., Centers for Dis. Control, Nat. Inst. Occupational Safety Health. Cincinnatti, OH. Osweiler, G. D. 1986. Toxicology of rodenticides and bird toxicants. Pages 165-169 in R. W. Kirk, ed. Current veterinary therapy IX: small animal practice. W. B. Saunders Co., Philadelphia. Osweiler, G. D., T. L. Carson, W. B. Buck, and G. A. Van Gelder. 1985. Clinical and diagnostic veterinary toxicology, 3d ed. Kendall/Hunt Pub. Co., Dubuque, Iowa. 494 pp. Pinto, L. J., and P. J. Spear. 1980. Technical data for pesticides. Nat. Pest Control Assoc. Vienna, VA. 124. pp. Savarie, P. J. 1991. The nature, modes of action, and toxicity of rodenticides. Pages 589-598 in D. Pimentel, ed. CRC handbook of pest management in agriculture, vol. II. CRC Press, Inc., Boca Raton, Florida. Spencer, E. Y., ed. 1981. Guide to the chemicals used in crop protection, 7th ed. Agric. Canada, Res. Branch, Pub. 1093. 595 pp. Sweet, D. V., ed. 1993. Registry of toxic effects of chemical substances, January 1993. Prepared for the Nat. Inst. Occupational Safety and Health by CAELUM Res. Corp., Silver Spring, Maryland. US Dep. Health Human Serv., NIOSH Publ. No. 93-101-2. Microfiche. Worthing, C. R., ed. 1991. The pesticide manual: a world compendium, 9th ed. British Crop Prot. Counc., Farnham, Surrey, United Kingdom. Editors Scott E. Hygnstrom Robert M. Timm Gary E. Larson G-61 G-62 Blaine (Jess) Benson POISON CONTROL CENTERS The Poison Center Children’s Hospital Omaha, Nebraska 68114 Poison control centers (poison centers) are an excellent starting point in the event of a pesticide mishap. The specially trained nurses, pharmacists, and physicians who staff these 24-hour emergency telephone services assess the severity of the pesticide exposure, make treatment recommendations, and often provide valuable information for spill containment and cleanup. When hospital treatment is required, the poison center contacts the receiving hospital and provides the emergency department with the most current treatment information available. During large-scale pesticide accidents (hazardous material incidents) many poison centers assist first responders with selection of proper protective gear, determination of proper decontamination techniques, and development of triage guidelines for victims. There is no charge for using a poison center. Usually state, county, or city taxes support this public service. In some states private hospitals or industries sponsor the program. Access to poison control centers varies across the United States. A current list of centers and their telephone numbers accompanies this section. When in doubt, readers should consult the front of their telephone book for the number of their poison control center or should contact their local telephone information operator. There is no single poison center designated for the United States. Instead, a regional network of poison centers exists. The advantage of this arrangement is that local poison centers know the capabilities of hospitals and first response crews in their region and are better able to guide poison victim treatment. When changes occur within the service region, such as a hospital closure, the local poison center is quickly able to adapt to the change. Not all poison centers are equal. Thirty-six US poison centers have been credentialed as regional poison centers by the American Association of Poison Control Centers (AAPCC). In order to be designated as a regional poison center by AAPCC, the center must meet criteria regarding accessibility, population base, staff, qualifications, medical direction, public and professional outreach, protocol development, knowledge of service area capabilities, and data collection. In general, AAPCC regional poison centers serve larger population bases, have larger call volumes, and access more extensive information resources than nonregional centers. Not surprisingly, several studies suggest that AAPCC regional centers handle calls more proficiently than nonregional poison centers. Calling a poison center during a pesticide mishap will further broaden our understanding of pesticide toxicology. Most poison centers in the United States contribute their case experience to the Toxic Environmental Surveillance System (TESS), a national poisoning database developed and maintained by the American Association of Poison Control Centers. Each year, the American Journal of Emergency Medicine publishes an abstracted version of the TESS data so that the entire medical community can learn more about poisoning trends. With over 1.5 million poisonings being recorded on an annual basis, it is possible to use the data to precisely target poison prevention efforts, optimize treatments for specific poisonings, and identify problematic drugs, pesticides, and household products. In a sense, each poison center caller contributes to improved care for the next caller. For Additional Information Geller, R. J., J. G. Fisher III, J. D. Leeper, J. D. Tooson, and S. Ranganthan. 1990. Poison centers in America: how well do they perform? Vet. Hum. Toxicol.: 32:240-245. Thompson, D. F., H. L. Trammel, N. J. Robertson, and J. R. Reingan. 1983. Evaluation of regional and nonregional poison centers. N. Engl. J. Med.: 308:191-194. Editors Scott E. Hygnstrom Robert M. Timm Gary E. Larson PREVENTION AND CONTROL OF WILDLIFE DAMAGE — 1994 Cooperative Extension Division Institute of Agriculture and Natural Resources University of Nebraska - Lincoln United States Department of Agriculture Animal and Plant Health Inspection Service Animal Damage Control Great Plains Agricultural Council Wildlife Committee G-63 AMERICAN ASSOCIATION OF POISON CONTROL CENTERS Certified Regional Poison Centers ALABAMA University of California, Davis, Medical Center Regional Poison Control Center Regional Poison Control Center 2315 Stockton Blvd. Sacramento, CA 95817 Emergency Phones: (916) 734-3692 (800) 342-9293 (Northern CA only) The Children’s Hospital of Alabama 1600 7th Ave. S Birmingham, AL 35233-1711 Emergency Phones: (205) 939-9201 (800) 292-678 (AL only) (205) 933-4050 COLORADO Rocky Mountain Poison and Drug Center ARIZONA Arizona Poison and Drug Information Center Arizona Health Sciences Center, Rm. 3204-K 1501 N. Campbell Ave. Tucson, AZ 85724 Emergency Phones: (800) 362-0101 (AZ only) (602) 626-6016 Samaritan Regional Poison Center Teleservices Dept. N. 12th St. Phoenix, AZ 85006 Emergency Phone: (602) 253-3334 CALIFORNIA Fresno Regional Poison Control Center of Fresno Community Hospital and Medical Center 2823 Fresno St. Fresno, CA 93721 Emergency Phones: (800) 346-5922 (209) 445-1222 645 Bannock St. Denver, CO 80204 Emergency Phone: (303) 629-1123 DISTRICT OF COLUMBIA National Capital Poison Center Georgetown University Hospital 3800 Reservoir Rd., NW Washington, DC 20007 Emergency Phones: (202) 625-3333 (202) 784-4660 (TTY) FLORIDA The Florida Poison Information Center at Tampa General Hospital Post Box 1289 Tampa, FL 33601 Emergency Phones: (813) 253-4444 (Tampa) (800) 282-3171 (Florida) GEORGIA San Diego Regional Poison Center UCSD Medical Center 8925 200 W. Air Dr. San Diego, CA 92103-8925 Emergency Phones: (619) 543-6000 (800) 876-4766 (in 619 area code only) San Francisco Bay Area Regional Poison Control Center San Francisco General Hospital 1001 Potrero Ave., Bldg. 80, Rm. 230 San Francisco, CA 94122 Emergency Phone: (800) 523-2222 Santa Clara Valley Medical Center Regional Poison Center S. Bascom Ave. San Jose, CA 95128 Emergency Phones: (408) 299-5112 (800) 662-9888 (CA only) G-64 Georgia Poison Center Grady Memorial Hospital 80 Butler St. SE Box 26066 Atlanta, GA 30335-3801 Emergency Phones: (800) 282-5846 (GA only) (404) 616-9000 INDIANA Indiana Poison Center Methodist Hospital of Indiana 1701 N. Senate Blvd. Box 1367 Indianapolis, IN 46206-1367 Emergency Phones: (800) 382-9097 (IN only) (317) 929-2323 MARYLAND MISSOURI Maryland Poison Center Cardinal Glennon Children’s Hospital 20 N. Pine St. Baltimore, MD 21201 Emergency Phones: (410) 528-7701 (800) 492-2414 (MD only) Regional Poison Center 1465 S. Grand Blvd. St. Louis, MO 63104 Emergency Phones: (314) 772-5200 (800) 366-8888 National Capital Poison Center (DC suburbs only) Georgetown University Hospital 3800 Reservoir Rd., NW Washington, DC 20007 Emergency Phones: (202) 625-3333 (202) 784-4660 (TTY) MASSACHUSETTS Massachusetts Poison Control System 300 Longwood Ave. Boston, MA 02115 Emergency Phones: (617) 232-2120 (800) 682-9211 MICHIGAN Blodgett Regional Poison Center 1840 Wealthy, SE Grand Rapids, MI 49506-2968 Emergency Phones: (800) 632-2727 (MI only) (800) 356-3232 (TTY) Poison Control Center Children’s Hospital of Michigan 3901 Beaubien Blvd. Detroit, MI 48201 Emergency Phone: (313) 745-5711 MONTANA Rocky Mountain Poison and Drug Center 645 Bannock St. Denver, CO 80204 Emergency Phone: (303) 629-1123 NEBRASKA Blaine (Jess) Benson The Poison Center Childrens Hospital Omaha, NE 68114 Emergency Phones: (402) 390-5555 (Omaha) (800) 955-9119 (NE) NEW JERSEY New Jersey Poison Information and Education System 201 Lyons Ave. Newark, NJ 07112 Emergency Phone: (800) 962-1253 NEW MEXICO New Mexico Poison and Drug Information Center MINNESOTA Hennepin Regional Poison Center Hennepin County Medical Center 701 Park Ave. Minneapolis, MN 55415 Emergency Phones: (612) 347-3141 Petline: (612) 337-7387 (612) 337-7474 (TDD) Minnesota Regional Poison Center St. Paul-Ramsey Medical Center 640 Jackson St. St. Paul, MN 55101 Emergency Phone: (612) 221-2113 University of New Mexico Albuquerque, NM 87131-1076 Emergency Phones: (505) 843-2551 (800) 432-6866 (NM only) NEW YORK Hudson Valley Poison Center Nyack Hospital 160 N. Midland Ave. Nyack, NY 10960 Emergency Phones: (800) 336-6997 (914) 353-1000 Long Island Regional Poison Control Center Winthrop University Hospital 259 First St. Mineola, NY 11501 Emergency Phones: (516) 542-2323, 2324, 2325, 3813 G-65 New York City Poison Control Center N.Y.C. Dept. of Health 455 First Ave., Rm. 123 New York, NY 10016 Emergency Phones: (212) 340-4494 (212) P-O-I-S-O-N-S (212) 689-9014 (TDD) OHIO TEXAS North Texas Poison Center 5201 Harry Hines Blvd. Box 35926 Dallas, TX 75235 Emergency Phones: (214) 590-5000, (800) 441-0040 (Texas Watts) Texas State Poison Center Central Ohio Poison Center 700 Children’s Dr. Columbus, OH 43205-2696 Emergency Phones: (614) 228-1323 (800) 682-7625 (614) 228-2272 (TrY) (614) 461-2012 Cincinnati Drug and Poison Information Center and Regional Poison Control System 231 Bethesda Ave., M. L. 144 Cincinnati, OH 45267-0144 Emergency Phones: (513) 558-5111 (800) 872-5111 (OH only) OREGON The University of Texas Medical Branch Galveston, TX 77550-2780 Emergency Phones: (409) 765-1420 (Galveston) (713) 654-1701 (Houston) UTAH Utah Poison Control Center 410 Chipeta Way, Suite 230 Salt Lake City, UT 84108 Emergency Phones: (801) 581-2151 (800) 456-7707 (UT only) VIRGINIA Blue Ridge Poison Center Oregon Poison Center Oregon Health Sciences University 3181 S. W. Sam Jackson Park Rd. Portland, OR 97201 Emergency Phones: (503) 494-8968 (800) 452-7165 (OR only) Box 67 Blue Ridge Hospital Charlottesville, VA 22901 Emergency Phones: (804) 924-5543 (800) 451-1428 National Capital Poison Center (Northern VA only) PENNSYLVANIA Central Pennsylvania Poison Center University Hospital Milton S. Hershey Medical Center Hershey, PA 17033 Emergency Phone: (800) 521-6110 Georgetown University Hospital 3800 Reservoir Rd., N. W. Washington, DC 20007 Emergency Phones: (202) 625-3333 (202) 784-4660 (TrY) WEST VIRGINIA The Poison Control Center serving the greater Philadelphia metropolitan area West Virginia Poison Center One Children’s Center Philadelphia, PA 19104-4303 Emergency Phone: (215) 386-2100 3110 MacCorkle Ave. S. E. Charleston, WV 25304 Emergency Phones: (800) 642-3625 (WV only) (304) 348-4211 Pittsburgh Poison Center 3705 Fifth Ave. Pittsburgh, PA 15213 Emergency Phone: (412) 681-6669 RHODE ISLAND Rhode Island Poison Center 593 Eddy St. Providence, RI 02903 Emergency Phone: (401) 277-5727 G-66 WYOMING The Poison Center 8301 Dodge St. Omaha, NE 68114 Emergency Phones: (402) 390-5555 (Omaha) (800) 955-9119 (NE) SPECIMEN LABELS Compiled by Scott E. Hygnstrom This section contains specimen labels of various products used for controlling wildlife damage. Products included here were selected as examples of registered vertebrate pesticides. Space limitations make it impossible to include labels from every available product. Inclusion of trade names, proprietary products, or company names does not imply endorsement by the University of Nebraska Cooperative Extension, USDA-APHIS-Animal Damage Control, or the Great Plains Agricultural Council. Similarly, no discrimination is intended against products or companies not included. Since pesticide labels change frequently, be sure to obtain, read, and follow current label directions when using any pesticide. Check with appropriate federal and state authorities to find out if a particular product is registered in your state. CONTENTS Birds Frightening Agents 4-aminopyridine (Avitrol®, CORN CHOPS) Repellents Methyl anthranilate (ReJex-iTTM AG-36, TP-40, and AP-50) Polybutene ( TANGLEFOOT® Bird Repellent) Stupefying Agents Alpha-chloralose (ALPHA-CHLORALOSE—USDA-APHIS) Toxicants Fenthion (Rid-A-Bird® Perch 1100 Solution) Starlicide (PURINATM STARLICIDETM COMPLETE, COMPOUND DRC-1339 98% CONCENTRATE -Feedlots Pigeons, Gulls, Livestock Depredations — USDA-APHIS) Mammals Repellents Ammonium soaps of higher fatty acids (HINDER® DEER AND RABBIT REPELLENT) Capsaicin (MILLER® HOT SAUCE® ANIMAL REPELLENT) Denatonium saccharide (RO-PEL® ANIMAL, RODENT AND BIRD REPELLENT, RO-PEL® GARBAGE PROTECTORTM) Methyl nonyl ketone (BONIDE® DOGZIT Dog and Cat Repellent) Naphthalene (SudburyTM, Chaperone® SQUIRREL and BAT REPELLENT) Polybutene (EATON’S® 4 THE SQUIRRELTM REPELLENT) Putrescent whole egg solids (DEER-AWAY® Big Game Repellent) Thiram (Gustafson THIRAM 42-S Repellent, NOTT CHEW-NOT ANIMAL REPELLENT) Tobacco Dust (F & B® rabbit and dog chaser) Ziram (EARL MAY® RABBIT SCAT) PREVENTION AND CONTROL OF WILDLIFE DAMAGE — 1994 Cooperative Extension Division Institute of Agriculture and Natural Resources University of Nebraska - Lincoln United States Department of Agriculture Animal and Plant Health Inspection Service Animal Damage Control Great Plains Agricultural Council Wildlife Committee G-67 Toxicants Anticoagulants Brodifacoum (Talon®-G RODENTICIDE BAIT PACK PELLETS) Bromadiolone (Contrac® Ready-to-Use Place Pac, maki® MINI-BLOCK, PURINATM MOUSE-A-RESTTM PELLETS) Chlorophacinone (EATON’S® AC90TM FORMULA PLACE PACK RODENTICIDE, ROZOL® RAT AND MOUSE KILLER, ROZOL® POCKET GOPHER BAIT) Diphacinone (Ditrac® TRACKING POWDER, Ditrac® RAT & MOUSE BAIT, EATON’S® ALL-WEATHER BAIT BLOCKS®, Liqua-Tox II®,RAMIK® GREEN BAIT PACKS, Rodere Parafinized Rat Bait, EATON’S® ANSWERTM FOR THE CONTROL OF POCKET GOPHERS) Pivalyl (EATON’S®A-C50TM) Warfarin (Final® RAT & MOUSE BAIT, PURINATM RAT CONTROL PELLETS, RODEXTM BLOXTM-1) Other Toxic Baits Bromethalin (PURINATM ASSAULT RAT PLACE PACK) Cholecalciferol (Quintox® RAT & MOUSE BAIT) Sodium Cyanide (M-44 CYANIDE CAPSULES—USDA-APHIS) Sodium Fluoroacetate (SODIUM FLUOROACETATE (COMPOUND 1080) LIVESTOCK PROTECTION COLLAR—USDA-APHIS) Strychnine (0.5% STRYCHNINE S.R.O. POCKET GOPHER BAIT FOR USE IN BURROW BUILDERS— USDA-APHIS, PETERSEN’S Pocket Gopher Killer I, WILCO GOPHER GETTER AG BAIT) Zinc Phosphide (BONIDE® MOLETOX II, BONIDE® ORCHARD MOUSE BAIT, RIDALL-ZINCTM TRACKING POWDER, ROBAN II AG, ZINC PHOSPHIDE ON WHEAT FOR MOUSE CONTROL USDA-APHIS, ZINC PHOSPHIDE CONCENTRATE FOR MUSKRAT AND NUTRIA CONTROL— USDA-APHIS, Zinc Phosphide Prairie Dog Bait—USDA-APHIS, ZP® Rodent Bait AG, ZP® Rodent Bait Place Pac, ZP® TRACKING POWDER) Fumigants Aluminum Phosphide (DEGESCH Phostoxin®) Chloropicrin (CHLOR-O-PIC®) Methyl Bromide (BROM-O-GAS®) Gas Cartidge (THE GIANT DESTROYER®, GAS CARTRIDGE—USDA-APHIS, GAS CARTRIDGE FOR COYOTES—USDA-APHIS) Reptiles Repellents Sulfur (Dr. T’s SNAKE-A-WAY® SNAKE REPELLENT) Editors Scott E. Hygnstrom Robert M. Timm Gary E. Larson G-68 G-69 G-70 G-71 G-72 G-73 G-74 G-75 G-76 G-77 G-78 G-79 G-80 G-81 G-82 G-83 G-84 G-85 G-86 G-87 G-88 G-89 G-90 G-91 G-92 G-93 G-94 G-95 G-96 G-97 G-98 G-99 G-100 G-101 G-102 G-103 G-104 G-105 G-106 G-107 G-108 G-109 G-110 G-111 G-112 G-113 G-114 G-115 G-116 G-117 G-118 G-119 G-120 G-121 G-122 G-123 G-124 G-125 G-126 G-127 G-128 G-129 G-130 G-131 G-132 G-133 G-134 G-135 G-136 G-137 G-138 G-139 G-140 G-141 G-142 G-143 G-144 G-145 G-146 G-147 G-148 G-149 INDEX Chemical and Trade Names (All page numbers refer to Section G) 0,0-dimethyl-0-(4-[methylthio]-m-tolyl) phosphorothioate 0-aminobenzoic acid methyl ester 43 0-carbomethoxyaniline 43 1,2-0-(2,2,2-trichloroethylidene)-α-D-glucofuranose 41 35 2,4a,5,5a,7,8,15,15a,15b,15c-dechydro-4,6-methano-6H,14H-indolo (3,2,1-ij)oxepino(2,3,4-de)pyrrolo(2,3-h quinolin-14-one 54-55 2-(diphenylacetyl)-1,3-indandione 6, 26, 28, 29, 108, 109, 110, 111, 112, 113, 114 2-isovaleryl-1,3-indandione 5 2-[(p-chlorophenyl)phenylacetyl]-1,3-indandione 5, 26-28, 105, 106, 107 2-pivalyl-1-3-indandione 6, 26, 27, 29, 115 3-[3-(4’-bromo[l,l’-biphenyl]4-yl)-1,2,3,4-tetra-hydro-l-naphthalenyl]4-hydroxy-2H-l-benzopyran-2-one 5, 26-29, 101 3-[3-(4’-bromo[1,1’-biphenyl]-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxy-2H-l-benzopyran-2-one 5, 26-29, 102, 103, 104 3-(α-acetonylbenzyl)-4-hydroxycoumarin 7, 26-29, 116, 117, 118 3-chloro-4-methyl benzylnamine hydrochloride 52-53, 80-81, 82-83, 84 3-chloro-p-toluidine hydrochloride 52-53, 79, 85-86 4-aminopyridine 20, 21, 30-31, 69 4-the squirrel 94 8-methyl-n-vanillyl-6-nonenamide 34 9,10-seocholesta-5,7,10(19)-trein-3 betaol 1080 6, 37 49-51, 122 AC50 115 AC90 105 All-weather bait blocks 110 Alpha-chloralose 35, 74-77 Aluminum phosphide 4, 8, 10, 11, 12, 13, 19, 25, 138-139 Ammonium soaps of higher fatty acids 18, 19, 40, 87-88 Anise, oil of 15 Anticoagulants 26-29 Assault® 7, 33, 119 Avitrol® 20, 21, 30-31, 69 Benzyldiethyl [(2,6-xylylcarbamoyl) methyl] ammonium benzoate 38 Benzyldiethyl [(2,6-xylylcarbamolyl) methyl] ammonium saccharide 38, 90, 91 Bitrex® 16, 38 Bone tar oil 18, 32 Brodifacoum 5, 26-29, 101 Bromadiolone 5, 26-29, 102, 103 Bromethalin 7, 33, 119 [(bromo-4'-[biphenyl-1-1']-yl-4)3-tetrahydro-1,2,3,4-napthyl-1]3-hydroxy-4,2H-l benzothiopyran-2-one Brom-o-gas® 8, 44-45, 142-144 Bye-Deer® 40 26, 27, 29 G-151 Capsaicin 10, 11, 14, 16, 18, 19, 34, 89 Capsicum 21 Chaperone® 93 Chew-not 98 Chlorophacinone 3, 5, 26-28, 105, 106, 107 Chlor-o-pic® 8, 36, 140-141 Chloropicrin 8, 36, 140-141 Cholecalciferol 6, 37, 120 Contrac® 27, 102 Contrax-D® 26, 28, 29 Coumafuryl 26 CPTH 52-53 Deer-Away® 18, 39, 95 Denatonium benzoate 38 Denatonium saccharide 3, 8, 14, 15, 18, 21, 38 Detia® 25 Dexol Gasser® 42 Difethialone 26, 27, 29 Diphacinone 3, 6, 26, 28, 29, 108, 109, 110, 111, 112, 113, 114 Ditrac® 6, 108, 109 d-limonene 15 Dogzit 92 DRC-1339 20, 52-53, 80-81, 82-83, 84, 85-86 Eaton’s® Answer 3, 114 Fenthion 20, 41, 78 Final® 116 Fumarin® 26 Fumitoxin® 25 Gas cartridges 4, 8, 11, 12, 13, 16, 17, 19, 42, 145-146, 147, 148 Gastoxin® 25 Giant Destroyer® 42, 145-146 Gopher Gasser® 42 Gopher getter AG bait 125 Havoc® 27 Hinder® 18, 40, 87-88 Hot Sauce Animal Repellent® 10, 11, 14, 16, 18, 19, 34, 89 Larvacide® 36 Lindane 21 Liqua-Tox II 111 M-44 cyanide capsules 48, 121 Magic Circle® 18, 32 Maki® 5, 103 Methyl anthranilate 43, 70-72 Methyl bromide 8, 44-45, 142-144 Methyl nonyl ketone 15, 92 Moletox II 126 Mouse-a-rest 104 Naphthalene 14, 15, 17, 21, 46, 93, 99 Nitrochloroform 36 N-methyl-2,4-dinitro-N-(2,4,6-tribromophenyl)-6-(trifluoromethyl) benzenamine G-152 7, 33, 119 Orchard mouse bait 127 Peterson’s pocket-gopher killer I 124 Petroleum naphtemic oils 21 PhosTek® 25 Phostoxin® 8, 10, 11, 12, 13, 19, 25, 138-139 Pindone 6, 26, 27, 29 Pivalyl® 6, 26, 27, 29, 115 PMP® 5, 26 Polybutenes 14, 22, 73, 94 Putrescent whole egg solids 18, 39, 95 Quintox® 6, 37, 120 Rabbit Scat 100 Ramik® 6, 26, 28, 29, 112 Rampage® 37 Red squill 8, 47 ReJeX-iT® 43, 70-72 Rid-a-bird® 1100 20, 41, 78 Ridall-zinc 128 Roban II AG 129 Rodere parafinized rat bait 113 Ro-pel® 3, 8, 14, 15, 18, 21, 38, 90, 91 RoZol® 3, 5, 26-28, 106, 107 Scilliroside glycosides 47 Smoke’Em® 42 Snake-a-way® 149 Sodium cyanide 16, 48, 121 Sodium fluoroacetate 16, 49-51, 122 Sodium monofluoroacetate 49-51 Starlicide® 20, 52-53, 79 Strychnine 3, 19, 54-55, 123, 124, 125 Sulfur 22, 149 Talon® 5, 27, 29, 101 Tanglefoot® 73 Tetramethylthiuram disulfide 56, 96-97, 98 Thiram 10, 13, 18, 19, 56, 96-97, 98 Tobacco dust 15, 19, 99 Trichloronitromethane 36 Trounce® 33 Valone 26 Vengeance® 33 6, 37 Vitamin D3 Warfarin 7, 26-29, 116, 117, 118 Zinc phosphide 4, 8, 9, 11, 12, 57-59, 126, 127, 128, 129, 130-131, 132-133, 134, 135, 136, 137 Ziram 19, 60, 100 ZP® Rodent bait 136 ZP® Rodent bait AG 135 ZP® Tracking powder 137 G-153 G-154 INDEX Target Species (All page numbers refer to Section G) Bats Bears Beavers Birds Blackbirds 2, 17, 46, 93 34 2, 3, 38, 90 2, 20-22, 30, 38, 41, 43, 46, 54, 69, 70-72, 73, 74-77 20-22, 30, 43, 52, 69, 79, 80-81 Cats Chipmunks Coots Cowbirds Coyotes Crows 2, 15, 38, 90, 91, 92 2, 13 35, 74-77 20, 30, 43, 69, 71, 80-81 2, 16, 42, 48, 49, 121, 122, 148 21-22, 30, 43, 52, 85-86 Deer Dogs 2, 18, 32, 34, 38, 39, 40, 56, 87-88, 89, 90, 95, 96-97, 98 2, 15-16, 34, 38, 46, 48, 90, 91, 92, 99, 121 Elk 2, 18, 39, 95 Finches, house Foxes 21 2, 16, 48, 121 Geese Gophers, pocket Grackles Gulls 35, 43, 70, 71, 72 2, 3-4, 25, 26, 42, 54, 107, 114, 123, 124, 125, 126, 135, 136, 145-146, 147 20-22, 30, 43, 69, 80-81 20-22, 43, 52, 71, 72, 84 Hares Horned larks 2, 19 21 Magpies Marmots Mice, deer Mice, house Mice, meadow Mice, pocket Mice, white-footed Microtus spp. Moles Muskrats 52, 85-86 13, 25 2, 9-10 2, 5-8, 26, 33, 36, 37, 38, 44, 90, 91, 101, 102, 103, 104, 105, 106, 108, 109, 110, 111, 112, 115, 116, 117, 118, 120, 128, 136, 137, 138-139, 140-141, 142-144 9-10, 34, 57, 98, 127, 135 2, 9-10 2, 9-10, 130-131, 135 9-10, 129, 130-131, 135 2, 19, 25, 56, 126, 145-146 2, 11, 132-133 Nutria 2, 11, 57, 132-133 Peromyscus spp. Pheasants Pigeons Porcupines Prairie dogs 9, 10, 135 21, 43 20-22, 30, 35, 41, 52, 73, 74-77, 78, 82-83 2, 11, 89 2, 11, 25, 42, 57, 134, 135, 138-139, 147 G-155 Rabbits Rats, cotton Rats, kangaroo Rats, Norway Ravens Rodents 2, 19, 26, 34, 40, 46, 56, 60, 87-88, 89, 96-97, 98, 99, 100 2, 12, 57, 129, 135 2, 12, 135 2, 5-8, 26, 33, 36, 37, 90, 91, 101, 102, 103, 105, 106, 108, 109, 110, 111, 112, 113, 115, 116, 117, 118, 119, 120, 129, 136, 138-139, 140-141, 142-144, 145-146 2, 9, 57, 129, 135 2, 5-8, 25, 26, 33, 37, 38, 44, 47, 57, 90, 91, 101, 102, 103, 105, 106, 108, 109, 110, 111, 112, 113, 115, 116, 117, 118, 119, 120, 129, 135, 136 23, 52, 85-86 2, 5-8, 25, 26-29, 33, 36, 37, 42, 44, 54, 56, 96-97, 138-139, 140-141, 142-144 Skunks Snakes Sparrows, house (English) Squirrels, ground Squirrels, tree Starlings 2, 17, 42, 145-146 2, 22, 46, 149 20-22, 30, 41, 43, 69, 73, 78 2, 12, 25, 26, 42, 57, 135, 138-139, 145-146, 147 2, 14, 38, 46, 89, 90, 91, 93, 94 20-22, 30, 41, 43, 52, 69, 71, 73, 78, 79, 80-81 Voles 2, 9-10, 26, 34, 89, 127, 129, 130-131, 135 Waterfowl Woodchucks Woodrats 35, 43, 71, 72, 74-77 2, 13, 25, 42, 145-146, 147 2, 12 Rats, Polynesian Rats, roof G-156