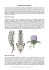wAMD Suppl_WebVersion_SinglePages_CMYK
Transcription
wAMD Suppl_WebVersion_SinglePages_CMYK
Supplement to For U.S. Audiences Only Supplement prepared and sponsored by EYLEA® (aflibercept) Injection is indicated for the treatment of patients with neovascular (Wet) Age-related Macular Degeneration (AMD). It is also indicated for the treatment of patients with Macular Edema following Central Retinal Vein Occlusion (CRVO). EYLEA is contraindicated in patients with ocular or periocular infections, active intraocular inflammation, or known hypersensitivity to aflibercept or any of the excipients in EYLEA. Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information Distributed with Neovascular (Wet) AMD and Anti-VEGF Therapy in Perspective In diagnosing and managing neovascular (wet) agerelated macular degeneration (AMD), retinal specialists must routinely address one of the greatest health fears of older patients – the potential for irreversible vision loss and blindness. Fear of vision loss outranks fear of cancer, heart disease, and stroke among adults.1 In less than a decade, the management of wet AMD has been transformed by intravitreal therapeutics that simultaneously inhibit aberrant vascularization and pathologic vascular leakage by blocking multiple isoforms of vascular endothelial growth factor-A (VEGF-A). Landmark studies demonstrated that pan-VEGF-A inhibitors could not only preserve visual acuity in the majority of patients with wet AMD, but could also produce clinically significant vision improvements in a substantial proportion of patients.2-5 Because these therapeutic agents are not curative, patient follow-up is ongoing and indefinite in order to maintain disease control.4 The challenge is to achieve the vision outcomes demonstrated by VEGF inhibitors in landmark clinical trials2-5 while appropriately striking a balance between the sight-threatening risks of injection-related complications (e.g., endophthalmitis and retinal detachment) and the sight-threatening risks of undertreating a progressive disease subject to unpredictable recurrences of retinal fluid. A substantial body of evidence has demonstrated that monthly office visits for monitoring and/or intravitreal injections have historically been needed in order to reliably achieve and maintain outcomes.4-8 Monthly visits for disease monitoring and/or intravitreal injections may not be a sustainable approach in the real world of clinical practice, as demonstrated by data from 2006-2008 for nearly 300,000 Medicare fee-for-service beneficiaries with newly diagnosed wet AMD. In this dataset in which nearly 95% of patients were treated with a pan-VEGF-A inhibitor, patients were seen by ophthalmologists an average of 7.1 times in the year following initiation of anti-VEGF therapy. Anti-VEGF therapy was discontinued within 1 year of initiation in more than half of treated eyes, although the barriers to care that would account for these findings could not be determined from the claims data.9 Monthly visits for monitoring/treating wet AMD can pose potential challenges in the management of wet AMD for patients and their caregivers. An observational evaluation was undertaken to understand the time commitment of just a single visit to monitor/treat wet AMD in real-world clinical practice. The average visit time for monitoring/treating wet AMD, based on patient record data (N=201), was ~90 minutes. In a separate component of the study, the total average time commitment for an appointment was ~3 hours (pre-appointment preparation, travel time, and in-office time) for patients undergoing anti-VEGF therapy; post-injection recovery time averaged 9 hours. Most patients (72%) were driven to appointments by someone else, generally the same person for all appointments. For the one-fourth of caregivers who had to take time from work to accompany patients, ~3 hours per visit would mean an annual time commitment for monthly wet AMD care equal to 1 week (i.e., 36 hours) of time off from work.10 Given the challenges of indefinitely sustaining a regimen of monthly monitoring injections, an unmet need was identified to achieve and maintain visual outcomes with less frequent office visits once therapy is initiated. Important Safety Information for EYLEA®(aflibercept) Injection Intravitreal injections, including those with EYLEA, have been associated with endophthalmitis and retinal detachments. Proper aseptic injection technique must always be used when administering EYLEA. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately. Intraocular inflammation has been reported with the use of EYLEA. Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information 1 Trap Technology and Aflibercept Cytokine traps11,12 such as aflibercept are molecules based on propriety technology developed at Regeneron Pharmaceuticals. In the case of aflibercept, the trap-like arms (Fig. 1) are each composed of the second extracellular ligand-binding domain from human VEGF receptor 1 (VEGFR-1) fused to the third extracellular ligand-binding domain from human VEGF receptor 2 (VEGFR-2)12 – domains critical for ligand contact and binding.13-15 Aflibercept therefore acts as a soluble decoy receptor, trapping VEGF-A and placental growth factor (PLGF) to prevent interactions with native cell-surface receptors.16 The two-arm trap configuration of aflibercept is supported by the constant Fc (Fragment, crystallizable) region of IgG1.12 In Fc-fusion proteins such as aflibercept, the Fc region is intended not only to stabilize the protein’s structure, but also to achieve an acceptable plasma half-life.17 However, other factors such as the receptor regions appear to be critical to determining the pharmacokinetics of a Fc-fusion protein.18 In vitro structure studies with aflibercept demonstrated the contribution of binding domains to pharmacokinetic activity. Aflibercept displayed negligible binding to extracellular matrix in vitro.12 The complex formed when aflibercept traps VEGF is inactive and stable. Studies of the relative serum concentrations of free aflibercept and aflibercept:VEGF complex following systemic administration in animal models have provided insight into the dynamics of endogenous VEGF intraocular blockade as it relates to intraviteal dosing and duration of VEGF blockade. These studies demonstrated that after drug administration, the aflibercept:VEGF complex accumulated rapidly as aflibercept trapped target ligands and free aflibercept was converted to bound complex. The level of aflibercept:VEGF complex plateaued at maximal concentrations when sufficient aflibercept was present to bind the total pool of endogeneous VEGF being produced by the tissues. With further increases in dose, free aflibercept concentrations increased in a dose-dependent manner since no more VEGF was available for binding with aflibercept. These observations suggested that the local VEGF pool would be completely neutralized as long as free aflibercept levels exceeded aflibercept:VEGF complex levels.10,19 CLEAR-IT Studies: Foundation for Intravitreal Aflibercept Injection Dosing Strategies Early phase clinical studies of intravitreal aflibercept injection (IAI), known commercially as EYLEA® (aflibercept) Injection, in patients with wet AMD identified dosing strategies for evaluation in pivotal Phase 3 studies. Single intravitreal aflibercept doses (0.05 to 4 mg) in the Clinical Evaluation of Anti-angiogenesis in the Retina Intravitreal Trial 1 (CLEAR-IT 1) in 21 patients provided initial evidence of the dose-dependent effects of intravitreal aflibercept injection. Higher single doses (2 and 4 mg) produced improvements in best-corrected visual acuity (BCVA) and choroidal neovascular (CNV) lesion morphology that were sustained for up to 6 weeks post-injection. With single doses of up to 4 mg intravitreal aflibercept, there were no reports of ocular, systemic serious adverse reactions, or laboratory changes. Intravitreal aflibercept injection was not associated with intraocular inflammation. In the absence of Figure 1. Trap structure of aflibercept based on preclinical studies. Aflibercept incorporates key domains of VEGFR-1 and VEGFR-2 in a homodimeric structure attached to the hinge region of the Fc domain of IgG1, which provides structural stability and an acceptable half-life in experimental models.12,17 VEGF dimer VEGFR1 binding domain 2 Aflibercept: VEGFcomplex VEGFR2 binding domain 3 Fc region IgG Aflibercept Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information 2 CLEAR-IT 2: Contributions to Intravitreal Aflibercept Injection Dosing CLEAR-IT 2 Observation21,22 Implication for Dosing Strategy ■ Greater BCVA improvements at 12 weeks after 3 monthly injections than after a single injection, a difference that was maintained throughout the remainder of the 52-week study ■ Therapy initiated with 3 monthly doses ■ Therapeutic effects of 2 mg and 4 mg did not differ ■ 2 mg as the maximum dose ■ Clinical effects (improved BCVA; decreased retinal thickness) following a single injection were sustained through 8 weeks and began to wane at 12 weeks ■ 8-week dose interval dose-limiting toxicity in this study, a maximum tolerated intravitreal aflibercept dose was not identified.20 wane at 12 weeks. Visual acuity improvements at 12 weeks were greater in patients who received 3 monthly injections than in those who received a single injection, a difference that was maintained throughout the remainder of the 52-week trial. Ocular adverse events (e.g., conjunctival hemorrhage, transient post-injection increase in intraocular pressure, refraction disorders, retinal hemorrhage, eye pain, vitreous detachment) were generally related to the injection procedure; most were mild. The results of CLEAR-IT 2 were the foundation of dosing protocols in Phase 3 studies and were incorporated into subsequent strategies for clinical use in wet AMD.21,22 Based on these findings, a subsequent Phase 2 dose-finding study (CLEAR-IT 2) examined the relative therapeutic effects of dose (0.5, 2, and 4 mg intravitreal aflibercept injection) and dosing interval (0.5 mg every 4 or 12 weeks; 2 mg every 4 or 12 weeks; 4 mg every 12 weeks) in order to define dosing strategies for evaluation in Phase 3 trials. In all groups, BCVA improved as early as 1 week after intravitreal aflibercept administration. Moreover, clinical effects following a single injection were sustained through 8 weeks and began to Figure 2. Design of VIEW 1 and 2 studies.23 In patients assigned to intravitreal aflibercept injection 2 mg every 2 months (2Q8), therapy was initiated with 3 monthly injections. Intravitreal aflibercept injection 0.5 mg every 4 weeks is not an approved dose. Multicenter, active-controlled, double-masked trials VIEW 1, N=1210; VIEW 2, N=1202 1:1:1:1 randomization Intravitreal Aflibercept Injection (IAI) Ranibizumab (RBZ) 0.5 mg every 4 weeks (RBZ 0.5Q4) 2 mg every 4 weeks (IAI 2Q4) 2 mg every 8 weeks (IAI 2Q8) 0.5 mg every 4 weeks (IAI 0.5Q4) Endpoints at 52 Weeks Primary Proportion (%) patients maintaining vision, ie, losing <15 letters BCVA vs baseline Additional Measures Mean change from baseline in BCVA Proportion (%) patients gaining ≥15 letters BCVA from baseline Anatomic measures of disease activity Important Safety Information for EYLEA®(aflibercept) Injection Acute increases in intraocular pressure have been seen within 60 minutes of intravitreal injection, including with EYLEA. Sustained increases in intraocular pressure have also been reported after repeated intravitreal dosing with VEGF inhibitors. Intraocular pressure and the perfusion of the optic nerve head should be monitored and managed appropriately. 3 Figure 3. Primary endpoint at 52 weeks for VIEW 1 and 2 studies separately and integrated.23 Bar chart on left reports proportion of patients who maintained visual acuity. Chart on right depicts noninferiority analysis for between-group differences in mean visual acuity change at 52 weeks. Last observation carried forward in the full analysis set. Although intravitreal aflibercept injection 0.5Q4 demonstrated similar efficacy and safety to intravitreal aflibercept injection 2Q4, it is not an approved dose and is not shown. Noninferiority Analysis† Proportion of Patients Who Maintained Visual Acuity VIEW 1 VIEW 2 Integrated 94% 95% 94% 95% 95% 95% 95% 95% 95% Statistically Noninferior VIEW 1 VIEW 2 % Patients IAI 2Q8* IAI 2Q4 RBZ Q4 291 306 309 IAI 2Q4 -0.3 -4.0 Integrated 304 304 302 0.6 -2.9 IAI 2Q8* 4.4 1.3 -2.4 IAI 2Q4 N 0.6 -3.2 IAI 2Q8* IAI 2Q4 Statistically Noninferior Clinically Equivalent 5.0 5.0 3.3 -1.7 0.9 3.5 -1.7 0.9 3.5 595 607 610 IAI 2Q8* -10 -5 0 RBZ 0.5Q4 5 10 IAI All intravitreal aflibercept doses noninferior to ranibizumab *Following 3 initial monthly doses (every 4 weeks) RBZ, ranibizumab IAI, intravitreal aflibercept injection VIEW 1 and VIEW 2 Studies: Intravitreal Aflibercept Injection Dosing Strategies in Wet AMD Defined The efficacy and safety of intravitreal aflibercept injection in wet AMD has been established in a robust worldwide program of Phase 3 randomized, double-masked, active controlled trials, VIEW 1 and VIEW 2 (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration) studies. These remain the largest randomized, double-masked controlled studies in wet AMD, involving ~2400 patients with predominantly classic, minimally classic, and occult neovascular AMD.23 Because ranibizumab 0.5 mg monthly had been previously proven to be effective in preventing clinically significant vision loss in wet AMD at the time of trial design,2,3 sham control in wet AMD studies would have been unethical.24 Therefore, ranibizumab served as the active control in the VIEW studies, with the control group ensured of receiving effective wet AMD standard of care based on the U.S. Prescribing Information for ranibizumab at the time of the studies. VIEW 1 was conducted in the United States and Canada; VIEW 2 was conducted in 26 countries worldwide. Equal numbers of patients were randomized to one of four treatment arms – ranibizumab dosed 0.5 mg every 4 weeks (0.5Q4) or intravitreal aflibercept injection dosed 2 mg every 4 weeks (2Q4), 2 mg every 8 weeks following 3 initial monthly injections (2Q8), or 0.5 mg every 4 weeks (0.5Q4) (Fig. 2). Fixed-dose regimens were administered for 52 weeks, the cutoff point for the primary efficacy analysis. The primary endpoint was proportion of patients who maintained vision, defined as losing <15 letters as measured by the ETDRS (Early Treatment Diabetic Retinopathy Study) protocol. Other efficacy measures included mean change in visual acuity, proportion of patients who gained ≥15 letters or ≥3 lines of vision, and the proportion of patients achieving ≥20/40 vision on the Snellen chart. In addition, changes in anatomic features, measured by time-domain optical coherence tomography (TD-OCT), compared the pharmacodynamic effects of the different treatment regimens. Although OCT examinations were performed throughout the study, they were not used to guide dosing decisions in the first 52 weeks during fixed-dose administration.23 Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information 4 Figure 4. A: Mean visual acuity change from baseline over 52 weeks (ETDRS letters). B: Anatomic effects measured as mean change from baseline in central retinal thickness (TD-OCT) over 52 weeks.10,23 Last observation carried forward in full analysis set. VIEW 1 TD-OCTs: Mandatory at baseline, Weeks 4, 12, 24, 36, 52. VIEW 2 TD-OCTs: Mandatory at all visits. Anatomic measures were not used to guide dosing decisions in the first 52 weeks of the VIEW 1/VIEW 2 studies. 15 A. VIEW 1 10.9* IAI 2Q4 (N=304) BCVA change from baseline, mean ETDRS letters 10 8.1 RBZ Q4 (N=304) 7.9✝ IAI 2Q8 (N=301) 5 0 15 VIEW 2 9.4 RBZ Q4 (N=291) 8.9✝ IAI 2Q8 (N=306) 7.5✝ IAI 2Q4 (N=309) 10 5 0 15 Integrated 9.3✝ IAI 2Q4 (N=613) 8.7 RBZ Q4 (N=595) 8.4✝ IAI 2Q8 (N=610) 10 5 0 0 4 8 12 16 20 24 28 32 36 40 44 48 Central retinal thickness, mean change from baseline, μm B. 25 52 *P = 0.005 Weeks ✝ P = N5 VIEW 1 -75 -117 IAI 2Q4 (N=304) -117 RBZ Q4 (N=304) -129 IAI 2Q8✝ (N=301) -125 -175 25 VIEW 2 -75 -139 RBZ Q4 (N=291) -149 IAI 2Q8✝ (N=306) -157 IAI 2Q4 (N=309) -125 -175 25 Integrated -75 -128 RBZ Q4 (N=595) -137 IAI 2Q4 (N=613) -139 IAI 2Q8✝ (N=610) -125 -175 0 4 8 12 16 20 24 28 32 36 40 44 48 52 Weeks RBZ 0.5Q4 IAI 2Q4 IAI 2Q8✝ ✝ Following 3 initial monthly doses (every 4 weeks) RBZ, ranibizumab IAI, intravitreal aflibercept injection Important Safety Information for EYLEA®(aflibercept) Injection The most common adverse reactions (≥5%) reported in patients receiving EYLEA were conjunctival hemorrhage, eye pain, cataract, vitreous detachment, vitreous floaters, and increased intraocular pressure. 5 Because the control group was active treatment, the VIEW 1 and 2 studies were designed as noninferiority studies to determine whether any of the intravitreal aflibercept injection dosing regimens were noninferior to ranibizumab. The statistical analysis used a confidence interval approach to assess the efficacy difference. In consultation and agreement with the U.S. FDA, the prespecified parameters for demonstrating noninferiority were a 95.1% (VIEW 1) or 95% (VIEW 2) confidence interval of the intravitreal aflibercept-ranibizumab difference and a 10% margin of difference, i.e., the upper limit of the confidence interval had to be <10% in order for intravitreal aflibercept to be proven noninferior to ranibizumab. Intravitreal aflibercept would be considered clinically equivalent to monthly ranibizumab if the upper confidence interval limit did not exceed 5% for the primary endpoint.23 Efficacy Outcomes Improvement in BCVA was noted as early as week 1 in all treatment groups, with an overall trend of continued improvement to the week 52 visit (Fig. 4A). Minor differences between groups disappeared when data were combined across studies, showing that the mean change in BCVA at week 52 was within 1 letter for all study treatments (8.4 to 9.3 letters). All intravitreal aflibercept groups, including the 2Q8 group, were similar to monthly ranibizumab.23 In the combined studies, most patients (79-81%) experienced some degree of visual acuity improvement at 1 year; 31-33% exhibited clinically significant improvement defined as ≥15-letter gain. A small subset (5-6%) showed ≥30-letter improvement. Compared with an average baseline Snellen score of 20/80, at least one-third of patients improved to ≥20/40 BCVA at week 52 (≤5% had 20/40 vision at baseline). For all of these secondary endpoints, clinical improvements were similar across study arms.10 At week 52, primary efficacy outcomes – proportion of patients in whom best-corrected visual Table 1. VIEW 1/VIEW 2 Studies: Most Common Adverse Events (≥1%) acuity was maintained (<15 letter loss on Over First 52 Weeks ETDRS chart from baseline to week 52) – were similar across groups, with vision Active Control IAI maintained in 94-95% of patients (Fig. 3). (RBZ, N=595) (N=1824) Intravitreal aflibercept injection met the % % prespecified statistical parameters for Conjunctival hemorrhage 28 25 being 1) noninferior to ranibizumab and 2) clinically equivalent to ranibizumab in Eye pain 9 9 maintaining vision after 52 weeks, with Cataract 7 7 confidence intervals for mean differences Vitreous detachment 6 6 falling within ⫾5%. Therefore, intravitreal Vitreous floaters 7 6 aflibercept injection administered as 2 mg every 8 weeks (following 3 initial monthly Intraocular pressure increased 7 5 doses) or as 2 mg every 4 weeks is clinically Conjunctival hyperemia 8 4 equivalent to ranibizumab 0.5 mg dosed Corneal erosion 5 4 every 4 weeks in terms of maintaining 23 Retinal pigment epithelium 3 3 vision in patients with wet AMD. These detachment outcomes were achieved with substantially fewer injections in the intravitreal Injection site pain 3 3 aflibercept injection 2Q8 group; patients Foreign body sensation in eyes 4 3 assigned to intravitreal aflibercept injection Lacrimation increased 1 3 2Q8 received an average of 7.5 active injections in VIEW 1 and VIEW 2, respectively Vision blurred 2 2 vs the 8-injection schedule specified by the Intraocular inflammation 3 2 protocol. For patients assigned to 0.5Q4 Retinal pigment epithelium tear 1 2 ranibizumab or intravitreal aflibercept Injection site hemorrhage 2 1 injection 2Q4, 13 injections were scheduled. The mean number of injections was Eyelid edema 2 1 12.3 for ranibizumab and intravitreal Corneal edema 1 1 aflibercept injection.25 RZB, ranibizumab IAI, intravitreal aflibercept injection Important Safety Information for EYLEA®(aflibercept) Injection Serious adverse reactions related to the injection procedure have occurred in <0.1% of intravitreal injections with EYLEA including endophthalmitis, traumatic cataract, increased intraocular pressure, and vitreous detachment. 6 Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information Anatomic Changes in the other arms.23 Post-hoc analyses examined whether these minor fluctuations reflected a small subset of patients in the intravitreal aflibercept injection 2Q8 arm who were having repeated episodes of larger fluctuations (e.g., >50 µm) with a negative impact on visual acuity. The analyses found that ~30% of patients in all treatment groups had such changes. These large fluctuations generally occurred once or twice during the study, although a small subset of patients had more frequent episodes. However, these fluctuations did not correlate with worse visual outcome at 52 weeks, even for patients who had several episodes in which central retinal thickness increased >50 µm, regardless of treatment.25 Anatomic measures were not used to influence treatment decisions. Changes in central retinal thickness as measured by optical coherence tomography (OCT) may generally be regarded as pharmacodynamic indicators of VEGF inhibition with regard to the reduction of fluid in/under the retina and retinal pigment epithelium (RPE) in wet AMD. Intravitreal aflibercept injection and monthly ranibizumab in both VIEW 1 and VIEW 2 had similar pharmacodynamic effects on these anatomic measures. Mean central retinal thickness was reduced by week 4, with reductions maintained through the end of year 1 (Fig. 4B). With OCTs being performed at all visits (monthly) in VIEW 2, minor inter-dose fluctuations in average central retinal thickness were observed in the intravitreal aflibercept injection 2Q8 group. The mean initial increase in retinal thickness was 17 µm between week 12 and week 16, decreasing steadily to 8 µm at week 52.23 These minor increases did not appear to have a negative impact on visual acuity in the overall group since mean visual acuity gains associated with intravitreal aflibercept injection 2Q8 were similar to those achieved Post-hoc analyses also examined the proportion of patients with a “dry” retina as defined by the masked reading center criteria of no retinal cysts/edema on TD-OCT. Across studies, groups were generally similar, with 62% of patients assigned to ranibizumab monthly and 72% (2Q4) and 68% (2Q8) receiving intravitreal aflibercept injection meeting the criteria for a “dry” retina.23 Similarly, no leakage on fluorescein angiography was observed in 61%, 71%, and 60%, respectively, when VIEW 1 and VIEW 2 data were combined.10 The correTable 2. VIEW 1/ VIEW 2 Studies: Serious Ocular and Nonocular (Systemic) lation of these anatomic Adverse Events Over First 52 Weeks* changes to visual outcomes is unknown. Active Control IAI (RBZ, N=595) (N=1824) Tolerability and Safety % % Any serious ocular adverse event 3.4 2.1 Visual acuity reduced 0.5 0.5 Retinal hemorrhage 0.5 0.3 Endophthalmitis 0.5 0.2 Cataract 0.2 0.2 Retinal pigment epithelium tear 0.2 0.2 Intraocular pressure increased 0.2 0.1 Retinal detachment 0.2 0.1 Macular hole 0 0.1 Posterior capsular opacification 0.3 0 Any serious non-ocular adverse event 14 14 3.5 2 Cardiac 3 3 Neoplasms 2 2.5 Vascular disorders 1 1 Injury/poisoning/procedural complications 1 2 Musculoskeletal/connective tissue 1 0.5 Gastrointestinal 1 1.5 Respiratory/thoracic/mediastinal 1 1 General/administration site 1 1 0.5 2 Infections/infestations Nervous system *Occurring in ≥2 patients in either group RZB, ranibizumab IAI, intravitreal aflibercept injection in Wet AMD Across the VIEW 1 and VIEW 2 studies, 1824 patients were exposed to intravitreal aflibercept injection (all doses combined) during the first 52 weeks; 595 were exposed to active control ranibizumab.23 Analysis of ocular and nonocular adverse events across intravitreal aflibercept injection dosage groups did not reveal a dose/exposure effect on tolerability or safety. The most common adverse events reported in ≥5% patients receiving intravitreal aflibercept injection were conjunctival hemorrhage, eye pain, cataract, vitreous detachment, vitreous floaters, and increased intraocular pressure (Table 1). The occurrence rates were similar for intravitreal aflibercept injection and active control. Hypersensitivity has been reported in <1% of patients 7 treated with intravitreal aflibercept injection. The overall incidence of serious ocular adverse events (Table 2) was similar between intravitreal aflibercept injection (2.1%) and active control (3.4%) groups. Serious adverse events reported in <1% of patients treated with intravitreal aflibercept injection were retinal detachment, retinal tear, and endophthalmitis. With intravitreal aflibercept injection, adverse events potentially related to systemic VEGF inhibition were uncommon and equally distributed among treatment groups (Table 2). Because VEGF-A helps regulate vascular tone, increased blood pressure is believed to be one of the most sensitive biomarkers of endogenous VEGF-A inhibition systemically.26 In the VIEW 1 and VIEW 2 studies, mean systolic blood pressure decreased from baseline over 52 weeks in all treatment arms; mean diastolic pressures also decreased slightly. Although mean blood pressure did not increase in the VIEW 1 and VIEW 2 studies, hypertension was reported as an adverse event along with reports of hypertension as serious adverse events; incidences were similar across intravitreal aflibercept injection and ranibizumab groups. Using the Antiplatelet Trialists’ Collaboration (APTC) criteria for classification of arterial thromboembolic events (ATEs) defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause),27 the incidence of ATEs with intravitreal aflibercept injection in clinical trials was 1.8% during the first year and 1.5% with active control. The Role of Intravitreal Aflibercept Injection in Wet AMD Management The progressive and often rapid visual acuity loss associated with wet AMD highlights the need for early diagnosis and effective treatment. Clinical studies have demonstrated that therapeutic proteins broadly targeting ligands of VEGFR-1 and VEGFR-2 can stabilize and even reverse vision loss in wet AMD, 2,3 making pan-VEGF-A inhibition a current standard of care in wet AMD. To reliably achieve and maintain these outcomes, patients (and caregivers) have historically had to follow a regimen of monthly office visits.6-8 Numerous studies have explored alternative treatment/monitoring regimens in order to reduce the frequency of monitoring visits and injections, thus suggesting a need for additional therapies. Aflibercept is a fusion protein containing all human amino acid sequences that serves as a decoy receptor to trap the VEGFR-1 and VEGFR-2 ligands VEGF-A and PLGF and inhibit receptor signaling.12 Formulated as an iso-osmotic solution compatible with the intraoacular environment, intravitreal aflibercept injection is a purified and formulated preparation of aflibercept, specifically for intravitreal injection.16 Intravitreal aflibercept injection is produced in recombinant Chinese hamster ovary (CHO) cells. For the primary measure of proportion of patients losing <15 EDTRS letters of best-corrected visual acuity from baseline, intravitreal aflibercept injection therapy allows the injection interval to be extended in wet AMD to 8 weeks following 3 initial monthly injections, as demonstrated by the VIEW 1 and VIEW 2 studies. In these studies, intravitreal aflibercept injection administered as 2 mg every 2 months (8 weeks) following 3 monthly (4 weeks) injections was statistically noninferior and clinically equivalent to ranibizumab monthly.23 The most common adverse events (≥5%) seen in the VIEW studies in patients treated with intravitreal aflibercept injection were conjunctival hemorrhage, eye pain, cataract, vitreous detachment, vitreous floaters, and increased intraocular pressure. Important Safety Information for EYLEA®(aflibercept) Injection There is a potential risk of arterial thromboembolic events (ATEs) following use of intravitreal VEGF inhibitors, including EYLEA, defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause). The incidence of ATEs in the VIEW 1 and VIEW 2 wet AMD studies in patients treated with EYLEA was 1.8% during the first year. The incidence of ATEs in the COPERNICUS and GALILEO CRVO studies was 0% in patients treated with EYLEA compared with 1.4% in patients receiving sham control during the first six months. 8 Please see Important Prescribing and Safety Information on back cover and the accompanying full Prescribing Information References 1. American Foundation for the Blind. American Foundation for the Blind Survey: Americans fear impact of vision loss more than cancer,HIV/AIDS, heart disease and stroke. 2007. http://www.prnewswire.com/news-releases/american-foundation-for-the-blind-launches-web-site-to-help-people-with-visionloss-maintain-independence-57786412.html Accessed July 15, 2013 2. Brown DM, Kaiser PK, Michels M et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New Engl J Med 2006;355:1432-44 3. Rosenfeld PJ, Brown DM, Heier JS et al. Ranibizumab for neovascular age-related macular degeneration. New Engl J Med 2006;355:1419-31 4. Martin DF, Maguire MG, Fine SL et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012;2012:26 5. CATT Research Group, Martin DF, Maguire MG et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New Engl J Med 2011;364:1897-908 6. Regillo CD, Brown DM, Abraham P et al. Randomized, doublemasked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008;145:239-48 7. Boyer DS, Heier JS, Brown DM et al. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular agerelated macular degeneration. Ophthalmology 2009;116:1731-9 8. Schmidt-Erfurth U, Eldem B, Guymer R et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: The EXCITE study. Ophthalmology 2011;118:831-9 9. Curtis LH, Hammill BG, Qualls LG et al. Treatment patterns for neovascular age-related macular degeneration: Analysis of 284 380 Medicare beneficiaries. Am J Ophthalmol 2012;153:1116-24 e1 10. Data on file. Regeneron Pharmaceuticals, Inc. 11. Economides AN, Carpenter LR, Rudge JS et al. Cytokine traps: Multi-component, high-affinity blockers of cytokine action. Nat Med 2003;9:47-52 12. Holash J, Davis S, Papadopoulos N et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 2002;99:11393-8 13. Davis-Smyth T, Chen H, Park J et al. The second immunoglobulinlike domain of the VEGF tyrosine kinase receptor FLT-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J 1996;15:4919-27 14. Lu D, Kussie P, Pytowski B et al. Identification of the residues in the extracellular region of KDR important for interaction with vascular endothelial growth factor and neutralizing anti-KDR antibodies. J Biol Chem 2000;275:14321-30 15. Wiesmann C, Fuh G, Christinger HW et al. Crystal structure at 1.7 a resolution of VEGF in complex with domain 2 of the FLT-1 receptor. Cell 1997;91:695-704 16. Papadopoulos N, Martin J, Ruan Q et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15:171-85 17. Czajkowsky DM, Hu J, Shao Z et al. Fc-fusion proteins: New developments and future perspectives. EMBO Mol Med 2012;4:1015-28 18. Suzuki T, Ishii-Watabe A, Tada M et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunology 2010;184:1968-76 19. Rudge JS, Holash J, Hylton D et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A 2007;104:18363-70 20. Nguyen QD, Shah SM, Browning DJ et al. A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration. Ophthalmology 2009;116:2141-8 e1 21. Brown DM, Heier JS, Ciulla T et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology 2011;118:1089-97 22. Heier JS, Boyer D, Nguyen QD et al. The 1-year results of CLEAR-IT 2, a Phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology 2011;118:1098-106 23. Heier JS, Brown DM, Chong V et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48 24. Food & Drug Administration. Guidance for industry: Non-inferiority clinical trials. March 2010. 25. Regeneron Pharmaceuticals Inc. FDA Briefing Document. Accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOp hthalmicDrugsAdvisoryCommittee/UCM259143.pdf 26. Jain RK, Duda DG, Willett CG et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol 2009;6:327-38 27. Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308:81-106. 9 IMPORTANT PRESCRIBING INFORMATION FOR EYLEA® (aflibercept) INJECTION EYLEA® (aflibercept) Injection is indicated for the treatment of patients with neovascular (Wet) Age-related Macular Degeneration (AMD). The recommended dose for EYLEA is 2 mg administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 2 mg once every 8 weeks (2 months). Although EYLEA may be dosed as frequently as 2 mg every 4 weeks (monthly), additional efficacy was not demonstrated when EYLEA was dosed every 4 weeks compared to every 8 weeks. EYLEA is indicated for the treatment of patients with Macular Edema following Central Retinal Vein Occlusion (CRVO). The recommended dose for EYLEA is 2 mg administered by intravitreal injection every 4 weeks (monthly). IMPORTANT SAFETY INFORMATION FOR EYLEA® (aflibercept) INJECTION EYLEA® (aflibercept) Injection is contraindicated in patients with ocular or periocular infections, active intraocular inflammation, or known hypersensitivity to aflibercept or to any of the excipients of EYLEA. Intravitreal injections, including those with EYLEA, have been associated with endophthalmitis and retinal detachments. Proper aseptic injection technique must always be used when administering EYLEA. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately. Intraocular inflammation has been reported with the use of EYLEA. Acute increases in intraocular pressure have been seen within 60 minutes of intravitreal injection, including with EYLEA. Sustained increases in intraocular pressure have also been reported after repeated intravitreal dosing with VEGF inhibitors. Intraocular pressure and the perfusion of the optic nerve head should be monitored and managed appropriately. There is a potential risk of arterial thromboembolic events (ATEs) following use of intravitreal VEGF inhibitors, including EYLEA, defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause). The incidence of ATEs in the VIEW 1 and VIEW 2 wet AMD studies in patients treated with EYLEA was 1.8% during the first year. The incidence of ATEs in the COPERNICUS and GALILEO CRVO studies was 0% in patients treated with EYLEA compared with 1.4% in patients receiving sham control during the first six months. The most common adverse reactions (≥5%) reported in patients receiving EYLEA were conjunctival hemorrhage, eye pain, cataract, vitreous detachment, vitreous floaters, and increased intraocular pressure. Serious adverse reactions related to the injection procedure have occurred in <0.1% of intravitreal injections with EYLEA including endophthalmitis, traumatic cataract, increased intraocular pressure, and vitreous detachment. Please see accompanying full Prescribing Information. ©2013, Regeneron Pharmaceuticals, Inc. 777 Old Saw Mill River Road, Tarrytown, NY 10591 All rights reserved 10/2013 LEA-0321