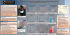Lactobacillus acidophilus Aeromonas hydrophila
Transcription
Lactobacillus acidophilus Aeromonas hydrophila
Egyptian Journal for Aquaculture Vol.2 No.2 2012 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus Mohammed E. Enany1, Mohamed E. Abou El-Atta2, and Mohamed M. El.Tantawy2 1- Microbiology Dept., Faculty of Veterinary Medicine, Suez Canal University. 2- Fish Health Dept., Central laboratory for aquaculture, Abbassa, Abou Hammad, Sharkia, Egypt. Abstract This study aimed to provide the in vitro effects of Lactobacillus acidophilus cell free extract and blue crab haemolymph serum for antagonizing growth of Aeromonas hydrophila and Vibrio alginolyticus isolated from diseased blue crab in comparison with florfenicol. Lactobacillus acidophilus was isolated from cattle milk whey. It was found that Lactobacillus acidophilus cell free extract and blue crab haemolymph serum had antibacterial effect against A. hydrophila and V. alginolyticus. The inhibition zones of bacterial growth were 12 and 16 mm in Lactobacillus acidophilus cell free extract discs; 15 and 12 mm in crab haemolymph serum discs and 25 and 22 mm in Florfenicol discs with A. hydrophila and V. alginolyticus, respectively, with a critical evaluation of results obtained. Key words: Lactobacillus acidophilus, alginolyticus, crab, antibacterial. Introduction Diseases of fish are major problems for the fish farming industry, which currently is the fastest growing food-protein producing sector with an annual increase of approximately 9%. Among those, bacterial infections are considered as the major cause of mortality in fish hatcheries (Grisez and Ollevier, 1995). Attention focused on the marine animals, production increased in the later seasons as it plays a role in environmental balance in marine aquaria. Crustaceans, such as crabs, shrimp and lobster, provide a high quality protein; also, it contains Aeromonas hydrophila, Vibrio Omega 3 fatty acids that afford potential health benefits (Wickins and lee, 2002). Bacterial strains were identical to Vibrio cholera. V. vulnificus, V. parahaemolyticus and others were routinely isolated from the haemolymph and external carapace of blue crab (Davis and Sizemore, 1982). The prophylactic and therapeutic controls of the bacterial diseases mostly begin in vitro, their application are based on the oral administration of antibiotics or by injection in narrow scale. However, such treatments may cause excess lost of drugs, and the development of resistant bacterial 63 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus strains (Aoki et al., 1985), yield residues in the animal and introduce potential hazard to public health and to the environment. Furthermore, the normal microbial flora in the digestive tract, which is beneficial to fish, may be killed or inhibited due to oral chemotherapy (Sugita et al., 1991). Therefore, the probiotics or haemolymph serum may be one of the factors of solutions. This work aimed to study the antibacterial activity of Lactobacillus acidophilus cell free extract and crab haemolymph serum for antagonizing A. hydrophila and V. alginolyticus bacteria in comparison with the antibiotic Florfenicol in vitro and the inhibition zone measured in mm. Materials and methods Isolation of Lactobacillus acidophilus from Cattle milk Lactobacillus acidophilus was isolated from cattle milk whey according to (Savadogo et al., 2004) with some modifications. Cattle milk was collected from the animal under aseptic condition in previously sterilized bottles and kept at room temperature till formation of crud, sieved and collected in sterile test tubes and incubated at 26oC for 72 hrs, since whey a suitable medium for the growth of its content of lactic acid bacteria. The isolates were platted out onto a specific broth medium, MRS (De Man, Rogosa and Sharpe, 1960) then streaked on MRS agar to obtain separate colonies; the pure colonies were kept on slope of MRS medium in 4oC for identification according to (Bergey et al., 2004). Preparation of Lactobacillus acidophilus cell free extract 64 Propagation was performed according to Ajitha, et al. (2004). Bacteria were grown aseptically in 10 ml of nutrient broth for 24 hrs at room temperature (28 ± 2°C). Five ml of log phase culture was then transferred under aseptic conditions into 250 ml of MRS broth and placed on a rotary shaker at 150 rpm for 24 hrs at 28 ± 2°C. The bacterial strain was harvested by centrifuging at 10,000 rpm under aseptic conditions for 15 min at 4°C. The cell free extract was saved, filtered and sterilized through Celtron filters of 0.2 mm pore size (Merck). PH adjusted to 6.8 by means of one mole NaOH. The cell free extract was stored at -70oC till use. Blue crab haemolymph serum Haemolymph was collected from blue crabs according to Noga et al. (1996 a&b) by inserting a 22 G needle attached to a 3 ml syringe into the arthrodial membrane of the swimming leg and gently aspirating into the syringe. The collecting haemolymph kept in Eppindorf tubes at 4oC till clot completed. Frozen haemolymph was kept at room temperature to allow homogenize and break up the clot. It centrifuged at 50,000 rpm for 20 min. The haemolymph serum was pooled and stored at -70oC until use. Bacterial strains Aeromonas hydrophilia and Vibrio alginolyticus were previously isolated from diseased blue crab. A swap from TSA (Treptic Soya agar) slants of bacterial strains under test (A. hydrophilia and V. alginolyticus,) were inoculated in nutrient broth (tube for each strain) and incubated at 28-30oC overnight then subculture on TSA Enany et al., medium supplemented with 0.5% sodium chloride. acidophilus isolated from Cattle milk was shown in Table (1), photo (1). Antibacterial activity The results revealed that Lactobacillus acidophilus cell free extract and blue crab haemolymph serum had antibacterial effect against Aeromonas hydrophila and Vibrio alginolyticus. This was obvious by a clear inhibition zone with no growth of bacteria around the test discs. This inhibition zones measured in mm and found as 12 and 16 mm in Lactobacillus acidophilus cell free extract discs; 15 and 12 mm in blue crab haemolymph serum discs, and 25 and 22 in Florfenicol discs, respectively, while normal saline impregnated discs showed bacterial growth in the entire circumference of TSA around the disc as shown in table (2) and photo (2) Agar disc diffusion method of Bauer et al (1996) was used for the assessment of antibacterial activity. Filter papers discs of 4 mm diameter were kept in aluminum foil placed in screw caped glass bottle and sterilized in autoclave; filter papers take three symbols (L, H and C) as abbreviations to Lactobacillus acidophilus cell free extract, blue crab haemolymph serum and control, while Florfenicol discs took the symbol (F). The sterilized filter paper discs with symbol (L) were impregnated with sufficient amount of Lactobacillus acidophilus cell free extract over night, discs with symbol (H) were impregnated with sufficient amount of blue crab haemolymph serum for 2 h, and discs with symbol C were impregnated with sufficient amount of sterile saline, (negative control). All discs were air dried under aseptic precautions. Florfenicol discs, Ffc 30 µ g was also used (positive control). Agar plates were prepared, one plate seeded with A. hydrophilia and the second with V. alginolyticus. Under aseptic conditions, on the surface of each plate, four discs were gently fixed (L, H, Ffc and C) and were incubated at 28-30oC overnight and the results were recorded. Antibacterial activity was expressed in terms of diameter of inhibition zone in mm. Inhibition was indicated by the absence of bacterial growth around the test discs and were obvious around the sterile disc. Results The biophysical and biochemical characteristics of Lactobacillus Discussion Probiotics are simply means “for life”, originating from the Greek words “pro” and “bios” (Gismondo et al., 1999). Today probiotics are quite commonplace in health promoting “functional foods” for humans, as well as therapeutic, prophylactic and growth supplements in animal production and human health (Mombelli and Gismondo, 2000; Ouwehand et al., 2002; Sullivan and Nord, 2002; Senok et al., 2005). The trial for controlling the pathogenic microorganisms was carried out by the use of safe, cheap, available, with no any harmful effect if it compared to that produced due to the chemotherapeutics use of drugs, it is the probiotic bacteria. By disc diffusion inhibition it was found that the inhibition zone diameter of lactic acid bacteria (Lactobacillus 65 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus acidophilus) isolated from cattle milk whey was found 12 and 16 mm against Aeromonas hydrophila and Vibrio alginolyticus, respectively. We conclude that Lactobacillus acidophilus was effective against the isolated microorganisms, first, by the antagonism to pathogens that shown in vitro. Second, by competition with pathogens for nutrients or for adhesion sites and this agree with the results performed by Ajitha et al. (2004) who studied the effect of Lactic acid bacteria (LAB) in vitro on some bacterial pathogens and cell free extracts of four strains of Lactic acid bacteria (LAB) viz. Lactobacillus acidophilus, Streptococcus cremoris, Lactobacillus bulgaricus –56 and Lactobacillus bulgaricus –57 found to be inhibitor for growths of Vibrio alginolyticus in nutrient broth. Antagonism of LAB against Vibrio alginolyticus confirmed by streak plating and suppression of growth of Vibrio was obtained. Shafiqur Rahman et al. (2009) studied the antibacterial activity of probiotic bacteria in vitro by the well diffusion assay where the cell-free extracts of 3day old cultures of candidate probionts were added in pre-formed wells, dug in Mueller-Hinton agar media and previously swabbed with target pathogens, revealed that a good number of probionts challenged the pathogens successfully as evidenced by the production of clear zones of inhibition against the growth of the target pathogens. Probionts isolated from Basal medium that selects the growth of Bacillus were found most efficient against Vibrios as they yielded 20-30 mm zones of inhibition. Nogami and Maeda (1992) rendered the antibacterial activity to the 66 production of extracellular products from these probiotic bacteria as rapid acidification through the production of organic acids, mainly lactic acid. In addition, their production of acetic acid, ethanol, aroma compounds, bacteriocins, exopolysaccharides, and several enzymes is of importance in Lactic acid bacteria (LAB), De Vuyst et al. (2004). Probiotics in aquaculture have been shown to have several modes of action: competitive exclusion of pathogenic bacteria through the production of inhibitory compounds; improvement of water quality; enhancement of immune response of host species and enhancement of nutrition of host species through the production of supplemental digestive enzymes (Thampson et al., 1999 and Verschuere et al., 2000). Inhibition of Vibrio by the cultures such as L. acidophilus, obtained in this study is in agreement with those obtained with LAB culture filtrate and by LAB in mixed culture against A. salmonicida (Gildberg et al., 1995). The haemolymph antibacterial activity as results of some material produced inside the humeral cells and released outside the cells as a defense mechanism against the invading microorganisms and its non specific in action, the same results were obtained by Noga et al. (1996 a&b). In general, crabs do not have a specific immune response (i.e. antibody or true lymphocytes) and instead rely on relatively non-specific broad-spectrum defenses such as phagocytosis, encapsulation and nonspecific defensive molecules (Fries, 1984). The defense mechanisms of crustaceans depend completely on the innate immune Enany et al., system that is activated when pathogen-associated molecular patterns are recognized by soluble cell surface host proteins such as lectins that were antimicrobial, clotting and pattern recognition proteins which in turn activate cellular or humoral effectors mechanisms to destroy invading pathogens (Vazquez et al., 2009). In crustaceans, circulating hemocytes play significant roles in the innate immune response, including release of non self-recognition proteins, clotting proteins, antimicrobial peptides and prophenoloxidase (So¨derha¨ll and Cerenius 1998; Terwilliger, 1999). Haemolymph of the brachyuran crustacean Callinectes sapidus possesses bactericidal activity which is highly inhibitory to Gram-negative bacteria cultured from blue crab carapace, including Aeromonas hydrophila, Vibrio parahemolyticus, V. alginolyticus and V. vulnificus. Several strains of Escherichia coli were also susceptible, no lysozymelike activity detected. The antibacterial activity appeared to be confined to the haemocytes. Initial investigations revealed that this antibacterial activity was proteinaceous (inactivated by proteolysis) and was found mainly within the hemocytes (Noga et al., 1996 a &b). Conclusion: It was found that chemotherapeutic materials has more potent antibacterial activity than natural materials since the control positive disc of Florfenicol was wider in diameter of inhibition against Aeromonas hydrophila and Vibrio alginolyticus ,while crab haemolymph serum antibacterial activities need more for studying as separation of its constituents by electric chromatography and study each band in a separate manner and decide which band would be more effective against several pathogenic bacteria then a biochemical analysis must be done to stand on its chemical composition before their use in a commercial. The use of probiotic materials as cell free supernatants of some bacteria as Lactobacillus acidophilus is easily to be obtained in a manufactured product and is safe for human and aquaculture as well as it does not play any role in mutations and resistance produced by the misuse of the whole bacterial cells. References Ajitha, S; Sridhar, M; Sridhar, N; Singh, I S B and Varghese, V (2004). Probiotic Effects of Lactic Acid Bacteria against Vibrio Alginolyticus in Penaeus (Fenneropenaeus) Indicus (H.Milne Edwards). Asian Fisheries Sci., 17: 71-80, Asian Fisheries Society, Manila, Philippines. Aoki, T; Kanazawa, T and Kitao, T (1985). Epidemiological surveillance of drug-resistant Vibrio anguillarum strains. Fish Pathol., 20: 199-208. Bauer, A W; M.Kirby, W M; Sherris, J C and Tuck, M (1996). Antibiotics Susceptibility testing by a Standardized single Disc Method Am. J. Clin. Pathol., 45: 493 – 496. Bergey, D; Sneath, P and John H (2004). Bergey’ s Manual of Systematic Bacteriology. Williams & Wilkins, Baltimore 67 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus The proteobacteria, part B, the gammaproteobacteria, pp.1-1106 Davis, J W and Sizemore, R K (1982). Incidence of Vibrio species associated with blue crabs (Callinectes sapidus) collected from Galveston Bay, Texas. Appl. Environ. Microbiol., 43:10921097. De Man, J C; Rogosa M and Sharpe, M E (1960). A medium for the cultivation of lactobacilli. J. Appl. Bacteriol., 23: 130-135. De Vuyst, L; Avonts, L and Makras, L (2004). Probiotics, prebiotics and gut health; in Remacle C, Reusens B (eds): Functional Foods, Ageing and Degenerative Disease. Cambridge, Woodhead Publishing, pp 416–482 Fries, C R (1984). Protein heamolymph factors and their roles in the invertebrate defense mechanisms. in: Cheng,T.C.(ed.). Comparative pathology. Plenum pres. New York, 6:49-1-9. Gildberg, A; Johansen, A and Bogwald, J (1995). Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquacul., 138: 23-34. Gismondo, M R; Drago, L and Lombardi, A (1999). Review of probiotics available to modify gastrointestinal flora. Intern. J. of Antimic. Agents, 12: 287–292. Grisez, L and Ollevier, F (1995). Vibrio (Listonella) anguillarum 68 infection in marine fish larviculture. In: Lavens, P., Jaspers, E., Roelande, l. (Eds.), Larvi 91-fish and crustacean larviculture symposium. Eur. Aquac. Soc. Gent., Special publication, 24: p 497. Mombelli, B and Gismondo, M R (2000). The use of probiotics in medicinal practice. Intern. J. of Antimic. Agents, 16: 531–536. Noga, E J; Arroll, T W and Fan Z (1996 a). Specificity and some physicochemical characteristics of the antibacterial activity from blue crab, Callinectes sapidus. Fish and Shell. Immun., 6: 403–412. Noga, E J; Arroll, T W; Bullis, R A and Khoo, L (1996 b). Antibacterial activity in the haemolymph of the white shrimp, Penaeus setiferus. J Mar Biotechnol., 4:181–184. Nogami, K and Meada, M (1992). Bacteria as bio control agents for rearing larvae of the Crab, Portunus trituberculatus. Canad. J. of fisher. and aquat. scie., 4(9): (2373-2376). Ouwehand, A C; Salminen, S and Isolauri, E (2002). Probiotics: an overview of beneficial effects. Antonie van Leewenhoek, 82: 279–289. Savadogo, A; Ouattara Cheik, AT; Bassole Imael, H N and Traore Alfred S (2004). Antimicrobial Activities of Lactic Acid Bacteria Strains Isolated from Burkina Faso Fermented Milk. Pakistan J of Nutri., 3 (3): 174-179. Enany et al., Senok, A C; Ismaeel, A Y and Botta, G A (2005). Probiotics: facts and myths. Clini. Microbi. And Infec., 11(12): 958–966. Terwilliger, N B (1999). Haemolymph proteins and molting in crustaceans and insects. Am. Zool., 39: 589–599. Shafiqur Rahman, S; Khan, N; Niamul Naser M and Manjurul Karim, M (2009); Application of probiotic bacteria: A novel approach towards ensuring food safety in shrimp aquaculture. J of Banglad. Acade. of Sci., 33(1): 139-144. Thompson, F L; Abreu, P C and Cavalli, R (1999). The use of microorganisms as food source for Penaeus paulensis larvae. Aquacul., 174: 139– 153. So¨derha¨ll, K., and L. Cerenius. (1998). Role of prophenoloxidaseactivating system in invertebrate immunity. Curr. Opin. Immunol. 10: 23–28. Sugita, H; Miyajima, C and Deguchi, Y (1991). The vitamin B12producing ability of the intestinal microflora of freshwater fish. Aquacult., 92: 267-276. Sullivan, A; Nord, C E (2002). The place of probiotics in human intestinal infections. Intern. J of Antimic. Agents, 20: 313–319. Vazquez, L; Alpuche, J; Maldonado, G; Agundis, C; Pereyra-Morales, A and Zenteno E (2009). Review: Immunity mechanisms in crustaceans, Innate Immu., 15 (3): 179-188. Verschuere, L; Rombaut, G; Sorgeloos, P and Verstraete, W (2000). Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev., 64: 655-671. Wickins, J F and Lee, D O C (2002). Crustacean Farming: Ranching and Culture, 2nd edn. Oxford, UK: Blackwell Science. 69 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus Table (1): Physical and biochemical characteristics of Lactobacillus acidophilus isolated from Cattle milk. Items Shape and arrangement Gram staining Motility Growth at 10oC Growth at 45oC Catalase test Growth in 4% NaCl Growth in 6.5% NaCl 0.3% methylene blue Reaction Rods, pairs chains Gram positive Non motile + + + + Items Fructose Glycerol Sucrose Maltose Lactose Mannitol Glucose Galactose Reaction + + + + + + Photo (1): Lactobacillus acidophilus Gram-positive reaction and bacilli shape from researching microscope using the oil-emersion lens (X= 1000). 70 Enany et al., Table (2): Inhibition zones (mm) diameters due to antibacterial activity of blue crab haemolymph serum, Lactobacillus acidophilus cell free extract and Florfenicol against A. hydrophila and V. anguillyseptica on TSA. Disc type Flofenicol disc Haemolymph serum disc Lactobacillus acidophilus Control (sterile saline) Inhibition zones diameter (mm) Aeromonas hydrophila Vibrio alginolyticus 25 22 15 12 12 16 0 0 Photo (2): In vitro antibacterial activity of Lactobacillus acidophilus cell free extract (L) and blue crab haemolymph serum (H) against Aeromonas hydrophila and Vibrio alginolyticus comprised with Florfenecol (F). C=control negative, A h= A. hydrophila and V a=V. anguillyseptica. 71 In vitro the effects of Lactobacillus acidophilus cell free extract and crab haemolymph serum as antagonizing Aeromonas hydrophila and Vibrio alginolyticus استخدام مستخلص الالكتوباسيلس اسيدوفيلس الخالى من الخاليا و سيرم هيموليمف الكابوريا ضد االيروموناس هيدروفيال و الفيبريوالجينوليتكس معمليا محمد السيد عنانى ، 1محمد السيد أبو العطا ، 2محمد مصطفى الطنطاوى 2 - 1قسم البكتريولوجى – كلية الطب البيطرى -جامعة قناة السويس. - 2قسم صحة ورعاية األسماك – المعمل المركزى لبحوث الثروة السمكية -العباسة الملخص العربى تهدف هذه الدراسة الى توفر استخدام البروبيوتك فى المعمل الستعداء بعض المعزولة من الكابوريا المريضة .لقد وجد أن مسضتخل مسضبباا االمضرال البكتيريضة الككتوباسضيلس اسضيدوفيلس المعزولضة مضن شضرل ألبضان الجاموس و الخالى من الخكيا و سيرم هيموليمف الكابوريا له نشاط مضاد لبكتريا االيرومونضاس هيضدروفيك و الفيبريوالجينضضوليتكس وتضضم قيضضاس قطضضر منطقضضة التثبضضي بضضالمم وكان ض 12و 11مضضم ف ض الككتوباسيلس اسيدوفيلس الخالى من الخكيا11،و 12مم فى اقرا فى اقرا أقضضرا مسضضتخل سضيرم هيموليمضف الكابوريضا و21و 22مضم الفلورفينيكول على التوال مع اجراء تقييم للنتائج الت تم الحصول عليها. 72