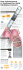Right diaphragmatic paralysis following endocardial cryothermal ablation of inappropriate sinus tachycardia
Transcription
Right diaphragmatic paralysis following endocardial cryothermal ablation of inappropriate sinus tachycardia
Europace (2006) 8, 904–906 doi:10.1093/europace/eul089 CASE REPORT Right diaphragmatic paralysis following endocardial cryothermal ablation of inappropriate sinus tachycardia Radu Vatasescu, Tchavdar Shalganov, Attila Kardos, Khatuna Jalabadze, Dora Paprika, Margit Gyorgy, and Tamas Szili-Torok* ¨rgy Hungarian Institute of Cardiology, Haller utca 29, H-1096, Budapest, Hungary Gottsegen Gyo Received 24 October 2005; accepted after revision 16 May 2006; online publish-ahead-of-print 3 August 2006 KEYWORDS Inappropriate sinus tachycardia (IST) is a rare disorder amenable to catheter ablation when refractory to medical therapy. Radiofrequency (RF) catheter modification/ablation of the sinus node (SN) is the usual approach, although it can be complicated by right phrenic nerve paralysis. We describe a patient with IST, who had symptomatic recurrences despite previous acutely successful RF SN modifications, including the use of electroanatomical mapping/navigation system. We decided to try transvenous cryothermal modification of the SN. We used 2 min applications at 2858C at sites of the earliest atrial activation guided by activation mapping during isoprenaline infusion. Every application was preceded by high output stimulation to reveal phrenic nerve proximity. During the last application, heart rate slowly and persistently fell below 85 bpm despite isoprenaline infusion, but right diaphragmatic paralysis developed. At 6 months follow-up, the patient was asymptomatic and the diaphragmatic paralysis had partially resolved. This is the first report, we believe, of successful SN modification for IST by endocardial cryoablation, although this case also demonstrates the considerable risk of right phrenic nerve paralysis even with this ablation energy. Introduction The potential for damage of right phrenic nerve during endocardial RF catheter modification/ablation of the sinus node (SN) for inappropriate sinus tachycardia (IST) is well known.1 This has been described during activationmapping-guided procedures2 as well as during procedures based on electro-anatomical mapping.3 We present a patient with symptomatic recurrence after each of the previous three endocardial radiofrequency (RF) catheter modifications of SN for IST. She underwent a further attempt at SN modification by endocardial cryothermal energy catheter applications. The procedure was guided by activation mapping and high-output pacing to avoid applications in the proximity of right phrenic nerve. This is the first report demonstrating that cryothermy can be used for SN modification in IST, although the risk of phrenic nerve paralysis still exists. Case report A 47-year-old woman was referred to our hospital because of permanent palpitation associated with fatigue and * Corresponding author. Tel: þ36 30 2 187637; fax: þ36 1 2151220/ext. 413. E-mail address: szili.torok@kardio.hu or szilitorok@chello.hu dyspnoea, and swelling of the lower extremities. She had normal coronary arteries, and all known cardiac and extracardiac causes of sinus tachycardia had been excluded during her clinical assessment. On multiple Holter ECG recordings, her average heart rate was always above 125 bpm. On exercise, she had sinus rate increase above 180 bpm. She had been diagnosed as having IST, which was refractory to multiple attempts at medical treatment including high doses of metoprolol, bisoprolol, betaxolol, verapamil, and sotalol, some of them in combination. Even amiodarone was tried unsuccessfully. During this period, her left ventricular function diminished to 45% (Simpson’s biplane method). In order to prevent further deterioration of her cardiac performance, she underwent three endocardial RF procedures for SN modification. The first two were guided by activation mapping, and ablation catheters with 4 mm tip (Celsius, Biosense Webster, Diamond Bar, CA, USA) and 8 mm tip (Stinger, Bard Electrophysiology, Lowell, MA, USA) respectively were used. The last one was electroanatomically guided and used a 3.5 mm externally irrigated tip ablation catheter (Navistar Thermo-Cool, Biosense Webster). Although each procedure was considered to be acutely successful, she had symptomatic recurrences within 1 month. Acute success with early recurrences raised the idea of inappropriate lesion formation. We & The European Society of Cardiology 2006. All rights reserved. For Permissions, please e-mail: journals.permissions@oxfordjournals.org Downloaded from by guest on October 28, 2014 Inappropriate sinus tachycardia; Endocardial catheter ablation; Cryothermal energy; Diaphragmatic paralysis Cryoablation of IST slight flattening of the P-wave in inferior leads (Figure 1), suggesting a shift in the dominant pacemaker to a more caudal position. However, after this last cryoenergy application during X-ray screening, there were obvious signs of right phrenic nerve paralysis: upward bulging and immobile right haemidiaphragm (Figure 2). Despite this, there was a sudden and marked symptomatic improvement. At 2 months follow-up, the patient remained asymptomatic although the right diaphragmatic paralysis had only partially regressed. At 4 months follow-up, the patient developed right-sided lobar pneumonia, with high fever. During this period, her heart rate did not rise above 160, with an acceptable average heart rate (110 bpm). At 6 months follow-up, she remained stable. Discussion Inappropriate sinus tachycardia is an uncommon rhythm disorder characterized by chronic and non-paroxysmal sinus tachycardia at rest and an exaggerated heart rate response to various stresses in the absence of secondary causes of sinus tachycardia. Endocardial RF catheter SN modification for IST despite a high rate of acute success of 70–100%4 has only a limited long-term effect, at best around 65%.4 This can be explained by the anatomy of the SN. Location of the SN is rather subepicardial in at least 70% of humans,5 and very often there is Figure 1 Surface electrocardiogram, intracardiac signals from mapping catheter and Holter ECG before (A), and after (C) successful cryoenergy application. Note the ‘far-field’ type appearance of the intracardiac signal at the site of earliest activation (A, arrow). Cryoenergy application produced a progressive slowing of heart rate as well as a slight flattening of the P wave in inferior leads and these changes persisted after ablation despite isoprenaline infusion (B). Downloaded from by guest on October 28, 2014 considered this as the effect of very cautious energy applications using RF energy. As stenosis of the superior caval vein and phrenic nerve damage has never been reported using cryothermy, we decided to perform endocardial cryothermal ablation for SN modification. To ensure appropriate lesion formation, we selected a new platform of cryoablation catheter using an 8 mm distal tip. Right ventricular apical back-up pacing and mapping/ablation (Freezor MAX, 9F, 8 mm tipped, CryoCath Technologies Inc., Montreal, Quebec, Canada) catheters were inserted via right femoral vein. Activation mapping at the superior vena cava (SVC)–right atrium (RA) junction was performed during isoprenaline infusion (2 mg/min). At sites of earliest activation, cryoenergy (2858C) was applied for 2 min, preceded every time by high-output bipolar pacing (15 mA, 3 ms) from the tip of the ablation catheter to reveal the possible proximity of the right phrenic nerve. An effective cryoenergy application was defined by a persistent heart rate fall below 100 bpm on isoprenaline infusion. Before any new cryoenergy delivery, a further activation mapping was performed. Ablation attempts were performed from superior to inferior along the SN region consistently with the benefit of previous experience.3 During the 12th application, a progressive heart rate fall was noted (from 100 bpm to 82 bpm at the end of cryoenergy application, Figure 1), which was persistent until the end of the procedure despite isoprenaline infusion. There was also 905 906 R. Vatasescu et al. Figure 2 (A) Chest X-ray after three RF ablations and before cryoenergy application. (B) Chest X-ray after cryoablation in deep inspiration. The right haemidiaphragm is paralysed. (C) Chest X-ray 6 months after cryoablation. There is marked improvement in right diaphragmatic paralysis. less than 2 mm distant from the anterior aspect of right superior pulmonary vein.13 To the best of our knowledge, this is the first case of successful endocardial SN modification for IST by cryoenergy. Moreover, this report confirms that although cryothermal lesions develop slowly, once the effect is complete, it can be long lasting. Therefore, the potential advantage of a deeper lesion had to be balanced over the risk for right phrenic nerve paralysis. References 1. Durante-Mangoni E, Vecchio DD, Ruggiero G. Right diaphragm paralysis following cardiac radiofrequency catheter ablation for inappropriate sinus tachycardia. Pacing Clin Electrophysiol 2003;26:783–4. 2. Man KC, Knight B, Tse H-F et al. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol 2000;35:451–7. 3. Marrouche NF, Beheiry S, Tomassoni G et al. Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia procedural strategies and long-term outcome. J Am Coll Cardiol 2002; 39:1046–54. 4. Shen WK. Modification and ablation for inappropriate sinus tachycardia: Current status. Card Electrophysiol Rev 2002;6:349–55. 5. Sa ´nchez-Quintana D, Cabrera JA, Farre ´ J, Climent V, Anderson RH, Ho SY. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 2005;91:189–94. 6. Ren J-F, Marchlinski FE, Callans DJ, Zado ES. Echocardiographic lesion characteristics associated with successful ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 2001;12:814–8. 7. Scherlag BJ, Yamanashi WS, Amin R, Lazzara R, Jackman WM. Experimental model of inappropriate sinus tachycardia: initiation and ablation. J Interv Card Electrophysiol 2005;13:21–9. 8. Mischke K, Stellbrink C, Hanrath P. Evidence of sinoatrial block as a curative mechanism in radiofrequency current ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 2001;12:264–7. 9. Mantovan R, Thiene G, Calzolari V, Basso C. Sinus node ablation for inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 2005;16:804–6. 10. Schweikert RA, Saliba WI, Tomassoni G et al. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation 2003;108:1329–35. 11. Koplan BA, Parkash R, Couper G, Stevenson WG. Combined epicardialendocardial approach to ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 2004;15:237–40. 12. Bonhomme CE, Deger FT, Schultz J, Hsu SS. Radiofrequency catheter ablation using non-contact mapping for inappropriate sinus tachycardia. J Interv Card Electrophysiol 2004;10:159–63. 13. Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? implications for cardiac interventionalists. J Cardiovasc Electrophysiol 2005;16:309–13. Downloaded from by guest on October 28, 2014 a thick muscular layer lying between endocardium and the SN, as well as a dense connective tissue matrix that embeds the nodal cells.5 There is also a ‘cooling’ effect of the nodal artery, which consistently penetrates the nodal body.5 Therefore, it is obvious that a successful RF endocardial ablation should produce a transmural lesion.6 A transmural lesion can be advantageous also if we consider the possibility of destroying autonomic ganglia localized in the fat pad of the terminal grove,7 which might be involved in the aetiology of the IST. However, transmural lesions can be sometimes difficult to achieve even with a large or irrigated tip due to lack of mural fixation of RF ablation catheters during cardiac movement. A non-transmural lesion can sometimes be acutely successful, inducing temporarily sino-atrial block,8 probably due to inflammation and tissue oedema.6 Recurrences are possible even with transmural lesions completely destroying SN,9 due to the widespread nature of the nodal tissue.5,9 In some patients, an epicardial approach guided by 3-D electroanatomical mapping is necessary for a successful ablation.10,11 The trade-off of producing RF transmural lesions is the risk of damaging the neighbouring structures to the heart. Sometimes this is manifest only as mild, transient pericarditis without effusion,12 but there is always a risk of cardiac perforation or right phrenic nerve paralysis.1,2,3,4 The latter is not uncommon and is easily explained by the fact that the nerve is separated only by pericardium at the anterolateral SVC–RA junction (the usual site for SN modification procedures).13 In our case, there were indications of the subepicardial location of the SN. The recurrences after endocardial RF procedures using large or irrigated catheter tips were suggestive. Another clue was the far-field aspect of the unipolar electrogram at the site of earliest activation11 (Figure 1A). For this reason, we chose endocardial cryoenergy for SN modification, hoping that increased catheter stability and a larger catheter tip would produce a deeper lesion. This was successful, however, at the price of right phrenic nerve paralysis despite the previous high-output pacing from the tip of the ablation catheter. There are only anecdotal cases that reported of complete or partial reversibility of right phrenic nerve paralysis with endocardial cryothermal energy ablation during pulmonary vein isolation procedures for atrial fibrillation. It is not surprising as in one-third of humans the right phrenic nerve is