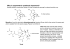REVIEW Chiral Toxicology: It`s the Same Thing...Only Different
Transcription
REVIEW Chiral Toxicology: It`s the Same Thing...Only Different
TOXICOLOGICAL SCIENCES 110(1), 4–30 (2009) doi:10.1093/toxsci/kfp097 Advance Access publication May 4, 2009 REVIEW Chiral Toxicology: It’s the Same Thing. . .Only Different Silas W. Smith*,†,1 *New York University School of Medicine, New York, New York 10016; and †New York City Poison Control Center, New York, New York 10016 Received February 18, 2009; accepted April 29, 2009 Chiral substances possess a unique architecture such that, despite sharing identical molecular formulas, atom-to-atom linkages, and bonding distances, they cannot be superimposed. Thus, in the environment of living systems, where specific structure-activity relationships may be required for effect (e.g., enzymes, receptors, transporters, and DNA), the physiochemical and biochemical properties of racemic mixtures and individual stereoisomers can differ significantly. In drug development, enantiomeric selection to maximize clinical effects or mitigate drug toxicity has yielded both success and failure. Further complicating genetic polymorphisms in drug disposition, stereoselective metabolism of chiral compounds can additionally influence pharmacokinetics, pharmacodynamics, and toxicity. Optically pure pharmaceuticals may undergo racemization in vivo, negating single enantiomer benefits or inducing unexpected effects. Appropriate chiral antidotes must be selected for therapeutic benefit and to minimize adverse events. Enantiomers may possess different carcinogenicity and teratogenicity. Environmental toxicology provides several examples in which compound bioaccumulation, persistence, and toxicity show chiral dependence. In forensic toxicology, chiral analysis has been applied to illicit drug preparations and biological specimens, with the potential to assist in determination of cause of death and aid in the correct interpretation of substance abuse and ‘‘doping’’ screens. Adrenergic agonists and antagonist, nonsteroidal anti-inflammatory agents, SSRIs, opioids, warfarin, valproate, thalidomide, retinoic acid, N-acetylcysteine, carnitine, penicillamine, leucovorin, glucarpidase, pesticides, polychlorinated biphenyls, phenylethylamines, and additional compounds will be discussed to illustrate important concepts in ‘‘chiral toxicology.’’ Key Words: antidotes; chiral; ecotoxicology; forensic toxicology; stereoisomer; teratology. 1 To whom correspondence should be addressed at Department of Emergency Medicine, Bellevue Hospital Center, 462 First Avenue, Room A-345A, New York, NY 10016. Fax: (212) 447-8223. E-mail: Silas.Smith@nyumc.org. This manuscript was developed from a presentation at the American College of Medical Toxicology Spring Conference, San Diego, CA, USA, March 2008. The author has no financial interest in any commercial products mentioned nor the companies that produce them. The use of trade names and/or commercial products in this review is for identification purposes only and constitutes neither a recommendation nor an endorsement for use by the author, the New York University School of Medicine, or the New York City Poison Control Center. No outside funding was received. As mechanisms of toxicity are elucidated, precise structureactivity relationships have become more apparent. This review introduces the concept of ‘‘chiral toxicology’’—the diverse impact of chirality on multiple aspects of toxicology. An historical overview and review of chemical principles guide an exposition of chiral illustrations in drug development, stereoselective metabolism and toxicity, chiral antidotes, carcinogenicity, reproductive toxicology, environmental toxicology, and forensic toxicology. HISTORY The study of chirality and symmetry has spanned the disciplines of chemistry, biology, and physics. Early work by Arago (1811) and Biot (1812a,b) demonstrated the effects of cut crystals on the plane of polarized light—different crystals rotated light left or right. Biot later showed that natural organic products, such as oil of turpentine, sugar solutions, camphor, and tartaric acid could produce similar rotation. In 1848, Pasteur recrystallized the racemic sodium ammonium salt of tartaric acid and noted two different crystal forms. Using tweezers, he separated them, and after redissolved them, found that they rotated polarized light differently (Pasteur, 1848). Subsequently, in 1857 Pasteur made the first observation of biological enantioselectivity, when he noted bacterial capacity to ferment only dextro-tartaric acid (Gal, 2008; Pasteur, 1857). In 1874, Van’t Hoff, recipient of the first Nobel Prize in Chemistry, and Le Bel independently outlined the relationship between three-dimensional molecular structure and optical activity and the concept of the asymmetric carbon atom (Le Bel, 1874; van’t Hoff, 1874). In 1891, Fisher performed the unbelievable feat of identifying the 16 different spatial configurations of aldohexose (C6H12O6), the most prominent member being D-glucose (Fischer, 1891). He created eponymous Fisher projections to represent their threedimensional structure (and was awarded the second Nobel Prize in Chemistry). The physiological and toxicological significance of chiral compounds was soon explored. Dextro-cocaine was found to Ó The Author 2009. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please email: journals.permissions@oxfordjournals.org CHIRAL TOXICOLOGY have greater activity and more rapid onset, and shorter duration than levo-cocaine (Ehrlich and Einhorn, 1894; Poulson, 1890). Differences in atropine (racemic hyoscyamine) and levohyoscyamine on pupillary, cardiac, and salivary activity and effects on frog spinal cord reflexes were described in 1903 (Cushny, 1903). Differences in toxic dose were reported for nicotine isomers in 1904 (Pictet and Rotschy, 1904) and for camphor isomers in 1910 (Grove, 1910). Exploring endogenous compounds, Abderhalden and Mu¨ller (1908) described the significant differential vasopressor effects of levo-and dextro-epinephrine. Therapeutically, Frey (1918) reported that quinidine, the isomer of quinine, was more effective in treating dysrhythmias. CHEMICAL PRINCIPLES Definitions and Terms Isomers are compounds with the same molecular formula but different structural formulas or different spatial arrangement (Fig. 1A). Methoxybenzene (anisole), phenylmethanol (benzyl alcohol), 2-methylphenol (ortho-cresol), 3-methylphenol (meta-cresol), and 4-methylphenol (para-cresol) are examples of constitutional or structural isomers. Although they share the same molecular formula (C7H8O) and molar mass, their atoms are linked differently (Fig. 1B), and they differ in physical properties. A chiral object contains an asymmetric point, often a carbon atom with different substituents, such that there can be two nonsuperimposable forms in three-dimensional space (Fig. 1C). Stereoisomers (spatial isomers) are compounds that share identical molecular formulas, atom-to-atom linkages, and bonding distances, but differ in their three-dimensional arrangement. Diastereomers are stereoisomers which are nonsuperimposable, non-mirror images. Enantiomers are stereoisomers which are nonsuperimposable, mirror images. They have identical physical and chemical properties except when they interact with chiral systems. Enantiomers rotate the plane of polarized light in opposite directions and by equal amounts. For example, levo- and dextro-ephedrine are enantiomers—they are mirror images, nonsuperimposable, and rotate light in opposite directions by equal amounts. Similarly, levo- and dextro-pseudoephedrine are enantiomers of each other. Pseudoephedrine and ephedrine are diastereomers. All contain the same number and type of atoms and bonding distances; all are ‘‘chiral’’ compounds. Cis-trans isomers are stereoisomers arising from compounds with restricted rotation (e.g., containing double bonds or some alicyclic compounds). Cis-diastereomers have substituent groups projecting in the same direction; trans-diastereomers have substituents oriented in opposing directions (Fig. 2A). Conformational isomers (conformers) are stereoisomers due to bond rotation. Acetylcholine is an example of a rotamer (conformer that differs by restricted rotation about only a single bond): the gauche forms are conformers of each other 5 (Fig. 2B) (International Union of Pure and Applied Chemistry [IUPAC], 1997). The 1,4-benzodiazepines typify ring inversion isomers. The seven-membered nonplanar ring can take on two possible ‘‘boat’’ formations that are conformational isomers of each other (Fig. 2C) (Paizs and Simonyi, 1999). Rotational barriers induced by large substituent groups may prevent rotation at low energy levels. Polychlorinated biphenyls (PCBs) are a good example of atropisomers— stereoisomers resulting from hindered rotation about single bonds, where the steric strain barrier to rotation is high enough to allow for the isolation of the separate conformers (e.g., Fig. 8C) (IUPAC, 1997). Several terms are commonly utilized to describe enantiomer proportions within a given mixture (Fig. 3). The enantiomer ratio (ER ¼ E1/E2) and enantiomer fraction [EF ¼ E1/(E1 þ E2)] convey similar information, but must be interpreted appropriately. The enantiomer excess [e.e. ¼ jE1 - E2j, where E1 þ E2 ¼ 1 (or 100%)] is defined as the absolute difference between the mole or weight fraction of each enantiomer, and is commonly expressed as a percentage (IUPAC, 1997). A racemate (racemic mixture) represents the unique case of a perfect 1:1 (equimolar, e.e. ¼ 0) mixture of enantiomers. Thus, it yields no optical rotation. Enantiomer proportions may change over time under certain experimental, physiological, or environmental conditions. Nomenclature Confusion arises from the multiple methods of naming chiral entities. The oldest d- (dextro, symbolized as ‘‘þ’’), l(levo, symbolized as ‘‘’’) terminology arose to describe how each specific enantiomer affected polarized light (Pasteur, 1848). When linearly polarized light passes through and interacts with an enantiomer sample, it undergoes ‘‘optical rotation.’’ If the enantiomer rotates the plane (of polarized light) to the right, it is termed dextro-rotary (þ). If it rotates the plane to the left, it is levo-rotary () (Fig. 4A). Enantiomers’ dextro- or levo-rotation are equal in absolute magnitude but opposite in direction. Importantly, the dextroor levo-rotation by each enantiomer has no correlation with the actual assignment of its atoms in space, and thus no relation to D/L or R/S naming conventions. For this reason, a compound’s (±) optical activity may be provided alongside its D/L or R/S name. Fisher developed the D/L system as a means to describe carbohydrate stereoisomers (Fischer, 1891). It is also commonly applied to amino acids. ‘‘D’’ or ‘‘L’’ is assigned by relating a molecule to (chiral) glyceraldehyde. The molecule is written in a Fisher projection. One aligns a given molecule with the carbon in the highest oxidation state superiorly. At the chiral center closest to the bottom, if the substituent (e.g., OH) projects to right, it is classified as the D-isomer. If the substituent projects to left, it is the L-isomer (Fig. 4B). The Cahn-Ingold-Prelog (CIP) System (R- [rectus], S- [sinister] convention) or ‘‘absolute configuration’’ is assigned by assigning ‘‘priority’’ to the 6 SMITH FIG. 1. (A) Classification of isomers. (B) Constitutional (structural) isomers of C7H8O. (C) A chiral structure with two nonsuperimposable forms (W 6¼ X 6¼ Y¼ 6 Z). groups attached to the stereocenter (by highest atomic number of the most proximate substituent and additional rules) (Cahn et al., 1956, 1966; Prelog and Helmchen, 1982). One then ‘‘looks down’’ the substituent with the lowest priority to determine if the decreasing order of the remaining groups occurs in a clockwise (R) or counterclockwise (S) fashion (Fig. 4C). R/S assignment does not always parallel D/L assignment. The E- (entgegen), Z- (zusammen) system describes more complex cis-trans configurations. CIP priorities are assigned to the substituents at each end of a rotational barrier. The (Z)-isomer has higher priorities groups on the same side; the (E)-isomer has two groups with the higher priorities on opposite sides (Fig. 4D). Endo- and exodesignations are applied to substituents of molecules which contain bridges. In endo-isomers, the group is towards (cisto) the longest bridge; exo-isomers are oriented away from the longest bridge (e.g., Fig. 7A). M (minus) and P (plus) designations for ring conformers such as diazepam are assigned on the basis of the characteristic torsion angle of C2-C3-N4-C5 of the diazepam ring (Fig. 2C) (Paizs and Simonyi, 1999). Confusingly, P (plus, right-handed, clockwise, D)- and M (minus, left-handed, counterclockwise, K) designations are also applied to supramolecular systems that are helical, propeller, or screw-shaped (Fig. 4E) (IUPAC, 1997). CHIRAL PHARMACOLOGY Drug Development FDA initial guidance on chiral drugs was set forth in 1992, as the differential actions and toxicities of enantiomers became more evident, and as the technology for chiral drug development and detection advanced (U.S. Food and Drug Administration, 1992). Identified chiral-specific issues included appropriate manufacturing controls (exclusion of diastereomeric impurities), product stability (racemization during storage), pharmacokinetic evaluations and quantifications that accounted for chiral differences (different dose-response curves), and correct interpretation of data when the pharmacokinetic properties of isomers in animal models differed from humans. According to the guidelines, composition of a chiral drug had to be known when applied in pharmacological, toxicological, and clinical studies. In part because of the burdens associated with determining the profiles and toxicities of mixed compounds, racemates have CHIRAL TOXICOLOGY 7 FIG. 2. (A) Cis-trans isomerism arising from the different position of atoms (or groups) around a reference point (platinum atom), a carbon-carbon double bond, and a cyclic structure (cycloalkane). (B) Acetylcholine conformers (rotamers) The gauche forms (seen in Newman projection) are conformers. (C) Two conformations of diazepam which interconvert via ring inversion. virtually disappeared from development as new molecular entities. Single enantiomer or achiral drugs now dominate newly approved drugs in the United States and abroad (Agranat et al., 2002; Caner et al., 2004) Additionally, drugs previously granted patent protection and marketed as racemates are candidates for a ‘‘chiral switch’’ (i.e., development as single or paired enantiomers (in the case of diasteromic mixtures), which permits additional years of market exclusivity (Agranat et al., 2002). Economic forces (e.g., market share) and favorable clinical profiles have driven successful chiral switches such as esomeprozole, levofloxacin, and escitalopram (Fig. 6A), and racemic veterinarian compounds (medetomidine) have been adapted for human use ((S)-dexmedetomidine) (Table 1). Antiviral analogues (abacavir, didanosine, lamivudine, stavudine, vidarabine, zidovudine, etc.) are produced as single isomers such that their ring substitutions mimic the 2R,5R architecture of the ‘‘natural’’ nucleosides (adenosine, cytidine, guanosine, thymidine, etc.). Compounds may contain atypical amino acid isomers or their derivatives (e.g., D-serine in goserelin). Other examples of current chiral pharmaceuticals from a range of pharmaceutical classes are provided in Table 1. These comprise drugs in which the enantiomer was developed primarily, ‘‘chiral switches,’’ and drugs offered only as enantiomers because the other isomer has no clinical effect or adverse effects in vivo (e.g., Levodopa and levothyroxine). Ideally, therapeutic activity would reside in one enantiomer and adverse effects in the other. Unfortunately, there is a range of possibilities, and the combined actions of the individual enantiomers may actually make the racemate or enantiomer combinations desirable. For example, when racemic dobutamine is administered, the nonselective b1 and b2 agonism by the l-dobutamine enantiomer is moderated by the a1 agonism of d-dobutamine to yield a clinical effect of apparent b1-selectivity (Majerus et al., 1989; Ruffolo, 1987). Alternatively, an individual enantiomer may retain the racemate’s undesirable activities, lose desired activity, or present new 8 SMITH FIG. 3. Distinctions between the terms used to convey enantiomer proportions. In a mixture composed of 75% of enantiomer 1 (E1) and 25% of enantiomer 2 (E2), the enantiomer ratio (ER ¼ E1/E2) ¼ 75%/25% ¼ 3.0, the enantiomer fraction [EF ¼ E1/(E1 þ E2)] ¼ 75%/(75% þ 25%) ¼ 0.75 (or 75%), and the enantiomer excess [e.e. ¼ jE1 E2j, where E1 þ E2 ¼ 1 (or 100%)] ¼ j75% 25%j ¼ 0.5 (or 50%). toxicities. R()-salbutamol (albuterol) is responsible for bronchodilator effects. S(þ)-salbutamol has little bronchodilating activity, actually causes hyperkalemia, and has been implicated in eosinophil activation and pro-inflammatory properties (Vakily et al., 2002). Unfortunately, R()-salbutamol (introduced as levalbuterol) retains the undesirable side effects of hypokalemia, hyperglycemia, tachycardia, and QT prolongation, and its clinical superiority is debated (Anonymous, 2006; Boulton and Fawcett, 1997; Vakily et al., 2002). Chiral drug development has produced several disappointments. Dexfenfluramine, an early chiral switch, retained the risk of pulmonary hypertension and valvular heart disease in combination with phentermine and was withdrawn along with racemic fenfluramine in 1997 (Anonymous, 1997). Dilevalol, the (R,R) stereoisomer of labetalol (which has two stereocenters), avoided postural hypotension due to its lack of alphaadrenergic receptor antagonism, but was associated with an increased risk of hepatotoxicity compared with labetalol and was not approved by the FDA (Scott, 1993; Walgren et al., 2005). Increased mortality in patients given d-sotalol—a class III anti-dysrhythmic essentially devoid of the beta-antagonism of the racemate—led to termination of the SWORD trial (Waldo et al., 1996). Development of the active isomer (R)fluoxetine ceased after dose-dependent QTc prolongation emerged in clinical trials, and its efficacy compared with racemic fluoxetine was questioned (Dorey, 2000). To complicate matters, chiral selection can be short-circuited and efficacy and toxicity data misinterpreted if a drug undergoes chiral inversion—the conversion of one enantiomer into its opposite (e.g., Fig. 7B) (Ali et al., 2007; Wsol et al., 2004). In vivo, this normally occurs with the assistance of an enzyme catalyst. Drugs from a variety of therapeutics classes that undergo chiral inversion have been reviewed (Ali et al., 2007; Wsol et al., 2004). Ibuprofen and other arylpropionic acid derivatives are well-described molecules capable of chiral inversion (Hao et al., 2005; Wsol et al., 2004). (S)-ibuprofen inhibits COX1 and COX2 equally. The (R)-enantiomer weakly inhibits COX1 and has no effects on COX2. However, an (R)metabolite, (R)-ibuprofenoyl-CoA, actually has greater COX2 inhibition. A majority of (R)-ibuprofenoyl-CoA undergoes unidirectional conversion to active (S)-ibuprofen (Knihinicki et al., 1991; Wsol et al., 2004). (R)-ibuprofen thus functions as a pro-drug and contributes to therapeutic effects (Neupert et al., 1997). In contrast, ketorolac (acetic acid derivative) displays species-specific interconversion—71% in mice and 12% in rats. Human inter-conversion is minimal—0% R(þ)-ketorolac to S()-ketorolac and 6% S()-ketorolac to R(þ)-ketorolac (Mroszczak et al., 1996). Thus, animal models might overestimate or underestimate efficacy if significant interconversion occurs. Age-dependent and enantiomer-specific elimination occurs with ketorolac—clearance in children is twice that of adults and clearance of active (S)-enantiomer is four times that of inactive (R)-isomer (Kauffman et al., 1999). This would suggest that higher weight-adjusted doses would be required to achieve comparable plasma concentrations of the active isomer in children. Nonstereospecific assays would also tend to misconstrue the duration of effect. Biological Implications: Metabolism and Clinical Effects Biological systems are chiral entities. Humans are primarily composed of L-amino acids and D-carbohydrates. Protein secondary structure includes right-handed (P-) coiled alpha helices, and most DNA is similarly in a right-handed spiral configuration (termed ‘‘B-DNA,’’ as opposed to left-handed ‘‘Z-DNA’’) (Fig. 4E) (Dickerson et al., 1982). Tertiary structures create unique three-dimensional binding, catalytic, and stabilization domains (Fig. 5). On the macroscopic scale, during normal growth and development both the human cardiac and gastrointestinal systems have specific rotational patterns, and additionally, left-right ‘‘mirror’’ symmetry evolves. Unique structural-activity relationships thus proceed from specific architecture constraints at multiple systemic levels. Therefore, in a chiral environment, stereoisomers might experience selective absorption, protein binding, transport, enzyme interactions and metabolism, receptor interactions, and DNA binding. Thus, each stereoisomer or isomeric mixture can have different pharmacokinetic, pharmacodynamic, therapeutic, and adverse effect profiles. A given structure’s capacity to accommodate chiral disparities will influence the magnitude and type of difference in effects (if any) observed between enantiomers. For example, one enantiomer might be completely unable to complex with a particular receptor or enzyme or lose precise alignment at a catalytic site, whereas at a different molecule, no impairment might occur (Fig. 5). These consequences of stereospecificity have been reported for multiple pharmaceutical classes including antibiotic, cardiovascular, chemotherapeutic, psychotropic, pulmonary, and rheumatic drugs (Baker and Prior, 2002; Hutt and O’Grady, 1996; Kean et al., 1991; Mehvar et al., 2002; Ranade and CHIRAL TOXICOLOGY 9 FIG. 4. Stereoisomer naming conventions. (A) According to optical activity: incoherent light is passed through a polarizer lens and then through a tube (polarimeter tube) containing a sample of the enantiomer. The degree of rotation of polarized light by an enantiomer is determined by an analyzer—a rotatable polarizer lens—in conjunction with a detector. Dextro-rotary (þ) compounds rotate the polarized light plane to the right (clockwise), levo-rotary (–) compounds to the left (anti-clockwise). (B) The d/l (Fischer) naming convention for glyceraldehydes—the asterix denotes the substituent determining D- or L-assignment. (C and D) The CIP R/S- and E/Z-naming conventions according to priority of substituents. (E) The IUPAC P,M-naming conventions of helical, propeller, or screw-shaped structures. Somberg, 2005; Vakily et al., 2002; Wainer and Granvil, 1993; Williams, 1990). Absorption/carriers. The drug efflux transporter Pglycoprotein, which participates in drug absorption, distribution, and excretion, is regulated stereospecifically. For example, R-cetirizine upregulates P-glycoprotein expression, while S-cetirizine down-regulates it (Shen et al., 2007). P-glycoprotein is enantioselectively inhibited by the levo-isomer of mefloquine, which can affect the transport of P-glycoprotein substrates such as cyclosporine and vinblastine (Lu et al., 2001; Pham et al., 2000). The human reduced folate carrier is stereospecific for the natural (6S) stereoisomer of 5-formyl tetrahydrofolate (leucovorin) and the antifolate methotrexate (Matherly and Hou, 2008; Narawa et al., 2007). Stereospecific transport contributes to methyl mercury central nervous system (CNS) toxicity. Methyl mercury binds cysteine to generate methyl mercury-cysteine [CH3-Hg-S-CH2-CH(NH2)COOH]. The structure’s mimicry of methionine [CH3-S-CH2-CH2CH(NH2)COOH] permits L-type large neutral amino acid 10 SMITH TABLE 1 Chiral Pharmaceuticals and Parent Racemates Isomer Armodafinil (Nuvigil) Betamethasone (many) Cisatracurium (Nimbex) Desloratadine (Clarinex) Dexamethasone (many) Dexchlorpheniramine (Polaramine, non-US) ˆ Dexmedetomidine (PrecedexO) Dexmethylphenidate (Focalin) Dextroamphetamine (Dexedrine) Dextromethorphan (many) Escitalopram (Lexapro) Esomeprazole (Nexium) Eszopiclone (Lunesta) Levalbuterol (Xopenex) Levobetaxolol (Betaxon) Levobunolol (Betagen) Levocabastine (Livostin) Levocetirizine (Xyzal) Levodopa (with carbidopa as Atamet, Sinemet) Levofloxacin (Levaquin) Levonorgestrel (Alesse, Seasonale, Plan B, etc.) Levorphanol (Levo-Dromoran) Levothyroxine (Synthroid, Levoxyl, etc.) Technetium Tc99m Bicisate (Neurolite) Racemate Modafinil (Provigil) N.A. (16S isomer of dexamethasone) Atracurium (Tracrium) Loratadine (Claritin) N.A. (16R isomer of Betamethasone) Chlorpheniramine (Chlor-Trimeton, etc.) Medetomidine (Dormitor, veterinary) Methylphenidate (Ritalin, etc.) Amphetamine (Adderall) N.A. Citalopram (Celexa) Omeprazole (Prilosec) Zopiclone (sold outside United States) Albuterol (Proventil, Ventolin, etc.) Betaxolol (Betopic) N.A. N.A Certirazine (Zyrtec) N.A. Ofloxacin (Floxin) Norgestrel (Lo/Ovral, Cryselle, etc.) N.A. N.A. N.A. carrier-mediated transport across the blood brain barrier (Clarkson et al., 2007). Methyl mercury-L-cysteine uptake significantly exceeds that of methyl mercury-D-cysteine (Kerper et al., 1992; Mokrzan et al., 1995). At certain concentrations, S()-bupivacaine has a vasoconstrictor effect absent in the R(þ)-isomer, which results in drug remaining at the injection site and a longer duration of analgesia (Aps and Reynolds, 1978). Levobupivicaine (Chirocaine), which did not carry the ‘‘black box’’ warning for cardiotoxicity required of racemic bupivacaine, has been discontinued in the United States (U.S. Food and Drug Administration, 2008). Protein binding. Chirality may influence the basic pharmacological property of protein binding. Albumin has speciesspecific, stereo-specific binding preferences (Pistolozzi and Bertucci, 2008). Despite diazepam’s rapid interconversion (Fig. 2C), its M-form prevails when bound to albumin. Bilirubin, which is achiral in solution due to rapid interconversion of its M- and P-forms, binds albumin in the P-form (Pistolozzi and Bertucci, 2008). Human albumin prefers the active S-enantiomer of ketoprofen and has stereoselectivity to other non-steroidal anti-inflammatory drugs (NSAIDs). Human albumin also displays stereoselective binding to FIG. 5. (A) An hypothetical enantiomer (Enantiomer1) interacting with a hypothetical enzyme structure (Target1), where domain A# is a catalytic site, B# is a hydrophobic pocket, and C# and D# are areas of steric hindrance. Enantiomer1 can effectively bind within the structure’s architecture and achieve appropriate orientation at the catalytic site. (B) Regardless of what rotational position Enantiomer2 assumes, it is incapable of appropriately aligning with either the active or stabilization domains (i.e., to avoid the steric hindrance imposed by D# on C, A is displaced away from the catalytic site A#, and B–B# interactions are not as efficient). (C) If a structure’s architecture is less constrained (e.g., Target2), then effective interactions may occur. warfarin (Fitos et al., 2002; Tatsumi et al., 2007). Albumin binds S(þ)-chloroquine more avidly than R()-chloroquine, whereas alpha-1-acid glycoprotein binds the R-enantiomer more tightly (Augustijns and Verbeke, 1993; Ofori-Adjei et al., 1986). Alpha-1-acid glycoprotein has stereospecific affinity for R()-disopyramide, S()-verapamil, and R(þ)-propranolol CHIRAL TOXICOLOGY (Hanada et al., 2000), and preferably binds the P-conformer of diazepam (Fitos et al., 2007). Correct interpretation of pharmacokinetic data and pharmacokinetic model constructs may be compromised if species-specific and enantiomerspecific differences in protein binding are not accounted for. The implications of stereoselective protein binding, bioequivalence, and the employment of stereospecific assay techniques are reviewed by Brocks (2006), Srinivas (2004), and others. In an organism provided a racemic ‘‘drug,’’ nonstereospecific assays would attribute an observed effect to the total free ‘‘drug’’ even if isomers had markedly dissimilar binding properties (e.g., protein binding of 65% in isomer R and 35% in isomer S would yield apparent ‘‘drug’’ binding of 50%). The correct clinical or adverse effect dose-response relationship would be misinterpreted if R/S isomer activity differed. As only free or unbound drug participates in elimination or receptor interaction, observed clearance, apparent volume of distribution, tissue distribution, and duration of effect might be further misconstrued, particularly as isomer ratios changed over time. Dose calculations and dosing intervals derived from a test species could be incorrect if humans or other species displayed alternative stereo-specific binding preferences (particularly of an active isomer) (Tocco et al., 1990). Thus, considering each stereoisomer as a distinct chemical entity refines pharmacokinetic models of racemates and mixtures. Metabolism and elimination. As might be expected for enzymatic processes, the biotransformation reactions (e.g., hydrolysis, reduction, oxidation, and conjugation) may demonstrate isomeric preference. (S,S)-hydroxybupropion is stereoselectively active at dopamine transporters, norepinephrine transporters, and nicotinic acetylcholine receptors (Damaj et al., 2004). At therapeutic concentrations, CYP2B6-mediated hydroxylation of (S)-bupropion to metabolically active (S,S)hydroxybupropion is significantly greater than (R)-bupropion, leading to greater apparent oral clearance and lower plasma concentrations (Kharasch et al., 2008). (S,S)-hydroxybupropion is formation-rate-limited, whereas (R,R)-hydroxybupropion and racemic hydroxybupropion are elimination-rate-limited; thus, CYP2B6 phenotypic variability, inhibition or induction, or overdose might alter the clinical consequences of bupropion ingestion (Kharasch et al., 2008). Hepatic, jejunal mucosa, and platelet sulfation of R()-salbutamol (albuterol) is approximately ten times greater than the S(þ)-isomer (Walle et al., 1993). The (S)-enantiomer of carvedilol undergoes stereoselective oxidation by cytochrome P450 (CYP) 2D6 and CYP1A2 in liver and stereoselective glucuronidation in liver and intestine, which is at least partly responsible for stereoselective presystemic clearance (Ishida et al., 2008). CYP2C19 preferential metabolism of S()-lansoprazole is further influenced by polymorphism status (homozygous and heterozygous extensive metabolizers, and poor metabolizers), such that the R/S ratios for the lansoprazole AUC in these polymorphisms is 12.7, 8.5, and 5.8, respectively, after oral dosing (Miura, 2006). 11 Similarly, systemic R/S enantiomer exposures to fluoxetine, metoprolol, pantoprozole, and trimipramine are altered according to CYP2D6 or CYP2C19 status (Brocks, 2006). CYP2D6 stereoselectively catalyses the O-demethylation of (R)venlafaxine (Eap et al., 2003). Stereoselective drug metabolism and elimination has been reported for a numerous other compounds: ketamine, whose R()-ketamine inhibits the more rapidly clearing S(þ)-ketamine; (S)-pentoxifylline conversion to its M1 metabolite; tramadol N-demethylation to S(–)-Odemethyltramadol; renal tubular secretion of dextro-cetirizine; and clearance of verapamil isomers (Garcia Quetglas et al., 2007; Ihmsen et al., 2001; Nicklasson et al., 2002; Schwartz et al., 1994; Strolin Benedetti et al., 2008; Williams and Wainer, 2002). From a therapeutic standpoint, proteaseresistant, cell-penetrating peptides are being developed for drug delivery through incorporation of D-amino acids (Pujals et al., 2008). Receptor (target) interactions. There are multiple examples of varied receptor types with chiral dependence. Vision itself requires cis-trans isomerism (Fig. 2A). 11-cis-retinal isomerizes to all-trans-retinal upon light absorption (Fig. 7C depicts analogous cis-trans isomers of retinoic acid in clinical use). This produces a conformational change within rhodopsin to yield activated metarhodopsin II, with subsequent triggering of the G-protein second messenger cascade and transmission of the photoactivation signal (Ahuja et al., 2009). Levo-carvone is perceived as spearmint, whereas dextro-carvone provides a caraway aroma (Williams and Wainer, 2002). R(þ)limonene, an essential oil employed as a food and cosmetic additive, solvent, and cleaning product generates a pleasant lemon-orange scent; S()-limonene smells like turpentine (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1999). Both may produce allergic reactions (Heydorn et al., 2003). Dextromethorphan primarily provides an anti-tussive effect, although levomethorphan, a noncommercially available DEA schedule 2 compound, has classic, potent systemic opioid effects. The conformers of acetylcholine (Fig. 2B) are physiologically relevant. It appears that the anti and gauche forms demonstrate variable affinity to nicotinic and muscarinic receptor subtypes, and the anti form is the preferential conformation for acetylcholinesterase catalysis (Edvardsen and Dahl, 1991; Lin et al., 2007; Vistoli et al., 2007). D-Serine coagonizes the N-methyl D-aspartate receptor (Wolosker et al., 2008). The antiepileptic S(þ)-vigabatrine irreversibly inhibits gamma- aminobutyric acid (GABA) transaminase, whereas the R-enantiomer is without activity (Gidal et al., 1999). Complicating matters, a xenobiotic may have stereospecificity at certain receptors, but not others (Fig. 5). S( )-propranolol exhibits approximately 100 times greater antagonism than R(þ)-propranolol at b1, b2, and b3 receptors (Popp et al., 2004; Ranade and Somberg, 2005). S()-propranolol is also up to four times more bioavailable and has a longer half-life, with stereoselective binding to human alpha 1-acid glycoprotein 12 SMITH (Walle et al., 1983). However, the ‘‘membrane stabilizing effect’’ and inhibition of thyroid hormone conversion are equal between the two enantiomers (Scott, 1993). Target selectivity also includes DNA. Transplatin (Fig. 2A), the isomer of the chemotherapeutic cisplatin, is without significant chemotherapeutic effect. This may be due to variable efficiency in forming DNA-protein cross-links, the different structure and stability of the DNA-protein adducts once formed, and solubility differences in aqueous media (Boudvillain et al., 1995; Kasparkova et al., 2008; Marchan et al., 2004) Chiral Pharmaceuticals: Clinical Toxicological Correlations Citalopram. Escitalopram, the active enantiomer, is a commercially successful chiral derivation from racemic citalopram (Fig. 6A). CYP 3A4 and CYP 2C19, and CYP 2D6 demethylate of escitalopram to (S)-desmethylcitalopram and (S)-didemethylcitalopram. There is wide inter- and intrapatient variability in escitalopram serum concentrations (Reis et al., 2007). Because of its serotonergic activity, escitalopram retains the ability to cause hyponatremia, serotonin syndrome, and restless leg syndrome (Covyeou and Jackson, 2007; Forest Pharmaceuticals, Inc. 2009; Grover et al., 2007; Huska et al., 2007; Nahshoni et al., 2004; Olsen et al., 2004; Page et al., 2008; Vari and Beckson, 2007) Both the S- and racemic citalopram prolong the QT equally and slightly more than placebo, and the manufacturer reports both QT prolongation and rare cases of torsade de pointes (Forest Pharmaceuticals, Inc. 2009). QT prolongation may be delayed for up to 10-h postingestion in escitalopram overdose, mandating prolonged cardiovascular monitoring (Yuksel et al., 2005). Thus, clinical superiority of escitalopram appeared undemonstrated in a review: despite the ability to take a lower absolute daily dose, the adverse effect profiles and cost are effectively similar (Anonymous, 2002). Methadone. Methadone use has been associated with prolonged QT interval and torsades de pointes (Ehret et al., 2006; Pearson and Woosley, 2005). Clinician unawareness of this risk led to pretreatment and ongoing electrocardiogram screening guidelines for patients prescribed methadone (Krantz et al., 2009). Opioid activity resides in the methadone R()enantiomer; temperature-dependent hERG potassium channel inhibition resides primarily with the S(þ)-enantiomer (IC50 of 2lM) (Fig. 6B) (Eap et al., 2007). Methadone is primarily metabolized by N-demethylation to an inactive metabolite EDDP (2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidene) (Gerber et al., 2004). In a complex fashion, stereoselective cytochrome P450 enzymes, primarily CYP3A4, CYP2B6, and CYP2C19, and to a lesser extent CYP2C9 and CYP2D6, convert methadone to EDDP and other inactive metabolites (Gerber et al., 2004; Totah et al., 2007). At CYP3A4, both R()- and S(þ)-enantiomers are metabolized equally, but when given as a racemate, there is mutual competitive inhibition, which results in equal decreased clearance. At therapeutic concentrations of each individual enantiomer, CYP2B6 FIG. 6. Examples of chiral pharmaceuticals—the asterix (*) denotes the chiral atom. (A) Racemic citalopram and escitalopram. (B) R- and Smethadone. (C) R- and S-warfarin. Upon ketoreductase reduction of the ketone (#) to warfarin alcohol, an additional chiral carbon is generated. metabolizes (S)-methadone faster; at higher concentrations, the (R)-enantiomer reaction proceeds faster. However, with the racemate, the (R)-enantiomer has virtually no effect on (S)methadone metabolism, where as (R)-metabolism is significantly retarded by (S)-, indicating stereo-preference for (S)-methadone. At CYP2C19, with less overall metabolism, just the opposite occurs, (R)- is more preferentially metabolized; again (S)- and (R)-inhibit each other’s metabolism. 2B6 genotype status—specifically the *6/*6 slow metabolizer—has been associated with a reduced ability to metabolize (S)methadone, a higher mean heart-rate–corrected QT, and an increased risk of prolonged QTc (Eap et al., 2007). A separate pharmacokinetic study evaluating peak and trough methadone levels in patients with various 2B6 genotypes also found (S)enantiomer concentrations could be achieved which could significantly impair repolarization (Crettol et al., 2005). Thus, genetic polymorphisms, coupled with dose-dependent stereochemistry might underlie the clinical toxicity seen with methadone administration. Warfarin. Warfarin has significant, divergent interpatient metabolism and dosing requirements. Patients display a wide dosing range to achieve the narrow therapeutic window CHIRAL TOXICOLOGY between overcoagulation (risking hemorrhagic complications) and undercoagulation (risking thrombotic complications). Warfarin is available only as a racemic mixture, although the S()-isoform is significantly more potent (Fig. 6C) (Scott, 1993). Metabolic hydroxylation occurs at various locations depending on the enantiomer (Au and Rettie, 2008; Kaminsky and Zhang, 1997; Park, 1988). S()-warfarin metabolism occurs via CYP2C9 (to 7-hydroxywarfarin, major contribution), CYP2C9 (to 6-hydroxywarfarin, moderate contribution); CYP3A4 (to dehydrowarfarin, minor contribution); and ketoreductase [to (S,S)-warfarin alcohol 2 minor contribution]. In comparison, R(þ)-warfarin metabolism proceeds by CYP1A2 (to 6- and 8-hydroxywarfarin, moderate contribution); CYP2C19 (to 8-hydroxywarfarin, moderate contribution); CYP3A4 (to 10-hydroxywarfarin, moderate contribution); CYP3A4 (to dehydrowarfarin, minor contribution); and ketoreductase [to (R,S)-warfarin alcohol 1, moderate contribution]. Suppression or acceleration of the various CYP enzymes involved by exogenous drugs can thus affect the clearance of both (R)- and (S)-isoforms. Moreover, because the (R)-enantiomer inhibits the metabolism of (S)-warfarin at CYP2C9, impaired metabolism of (R)-warfarin may cause increased levels of the active (S)-isoform. Complexity further increases when known genetic heterogeneity is superimposed upon interacting isomer and xenobiotic effects. CYP2C9 *2 and *3 variants cause 30% and 80% reduced activity and require lower warfarin doses. Additionally, a variable polymorphic pharmacodynamic contribution from the vitamin K epoxide reductase complex subunit (VKORC1) may also alter the R/S ratio and contribute to warfarin resistance (Au and Rettie, 2008; Osman et al., 2007; Rettie and Tai, 2006). The failure of a CYP2C9 and VKORC1-only genotype-guided warfarin dosing strategy to show benefit might in part be due to inattention to these additional stereospecific interactions (Anderson et al., 2007). CHIRAL ANTIDOTES Dextrose (D-glucose) Dextrose is a common antidote routinely administered to reverse hypoglycemia induced by organic or toxicological cause (anti-diabetics, ethanol, quinine, salicylates, etc.). Most glucose transporters, which are critical to growth, development, and health, have a high affinity for D-glucose, and generally negligible affinity for L-glucose (Fig. 4B, D/L conventions) (Cunningham et al., 2006). L-Glucose cannot be metabolized and does not show increased uptake in response to insulin in human volunteers. Other diverse downstream effects of D-glucose, such as upregulation of adenosine transport in aortic smooth muscle cells, decrease in lymphocyte intracellular ionized magnesium, and fibroblast premature replicative senescence do not occur if L-glucose is substituted (Blazer et al., 2002; Delva et al., 2002; Leung et al., 2005; MacLean 13 et al., 2001). In vitro, glucose isomers alter hematocrit and blood viscosity differently, due to erythrocyte uptake specificity for D-glucose (Buhler et al., 2001). Under conditions of excess solute-free water, D-glucose causes erythrocyte swelling, as water follows D-glucose intracellularly; under initial isotonic conditions, excluded L-glucose results in erythrocyte shrinkage. L-Glucose does taste as sweet as D-glucose, presumably by presenting the same glycophore (two-dimensional surface part) to the taste receptor (Shallenberger, 1997). However, L-glucose is a significant laxative, which is likely secondary to its limited oral and intestinal absorption (the three-dimensional structure conferring transporter specificity), with consequential induction of osmotic diarrhea (Fine et al., 1993; Kimura et al., 2002; Raymer et al., 2003). D-Glucose is also generally favored over other D-glucose epimers such as D-mannose or D-galactose. Similarly sodium-coupled glucose transporters show a much lower affinity for D-galactose than D-glucose (Scheepers et al., 2004). N-acetyl-L-cysteine (L-NAC) N-acetylcysteine provides an effective means of prevention and treatment of acetaminophen-induced hepatotoxicity, even in cases of delayed presentation following overdose (Keays et al., 1991; Smilkstein et al., 1988). L-NAC is also utilized to prevent contrast induced nephropathy (Massicotte, 2008). Only the Lform is antidotally useful. In animal experiments, the L-isomer, which is derived from physiologic L-cysteine, prevents hepatotoxicity and provides prolonged elevations of hepatic glutathione (Wong et al., 1986a). The nonphysiologic D-isomer cannot increase glutathione stores or prevent hepatotoxicity, despite increasing acetaminophen sulfation (Corcoran and Wong, 1986; Wong et al., 1986b), L-NAC also has demonstrable extra-hepatic benefits—improving cardiac index and systemic mean oxygen delivery despite decreasing systemic vascular resistance (Harrison et al., 1991). Interestingly, only the L-isomer was capable of mediating vascular tone—blunting tolerance to the hypotensive effect of glyceryl trinitrate in rats (Newman et al., 1990). L-NAC was twice as effective at inhibiting cell cycle progression and topoisomerase-IIa activity (Grdina et al., 1998). This would be protective by interrupting cell division in the setting of inadequate substrates or growth factors. L-NAC, but not D-NAC, provides protection against ultraviolet light induced DNA damage in cultured fibroblasts and retinal damage in vivo (Busch et al., 1999; Morley et al., 2003). The unnatural D-NAC also resists enzymatic degradation, limiting the useful liberation of cysteine (De Flora et al., 1995; Sarnstrand et al., 1995). L-Carnitine L-(R)-carnitine is primarily used in the treatment of valproate (VPA) toxicity (Lheureux et al., 2005; Russell, 2007). It is also suggested for treatment of drug-associated mitochondrial toxicity (e.g., from nucleoside analogs) and anthracycline 14 SMITH cardiotoxicity (Claessens et al., 2003; Delaney et al., 2007; Zeidan et al., 2002). Brain carnitine uptake is stereospecific for L-carnitine (Huth et al., 1981), and the acetylated L-form can serve as a precursor for releasable glutamate (Tanaka et al., 2003). Although both D- and L-carnitine reduce the incidence of murine ammonia-induced seizures, only L-carnitine lowered ADP and AMP levels (Matsuoka and Igisu, 1993). Compared with D-carnitine, L-carnitine also improved cardiac metabolic function, oxygen consumption, and mechanical efficiency by moderating free fatty acid metabolism (Liedtke et al., 1981, 1982). The D-isomer is considered biologically inactive and harmful (Hathcock and Shao, 2006). It competitively depletes serum and cardiac and skeletal muscles of L-carnitine (Arancio et al., 1989; Ayala, 1995; Rebouche, 1983; Tsoko et al., 1995), and competitively inhibits L-carnitine intestinal uptake and renal reabsorption (Gross and Henderson, 1984). Use of racemic D,L-carnitine was associated with myasthenialike syndromes (Bazzato et al., 1981; Clair et al., 1984; Rossini et al., 1981) and cardiac arrhythmias, which disappeared after L-carnitine administration. Toxic cardiac effects of D-carnitine have been described in patients with renal failure on long-term hemodialysis and in doxorubicin cardiotoxicity. Neither the D-isomer nor the racemate should be antidotally administered. Physostigmine Antidotal use of physostigmine (eserine) dates from 1864, when it was reported to reverse severe poisoning secondary to atropine ingestion (Nickalls and Nickalls, 1988). Physostigmine, a carbamate inhibitor, is derived from the seed (Calabar bean) of the vine Physostigma venenosum Balfour, and was used in the ancient trial by ordeal (Fraser, 1863). Although its nonspecific analeptic properties are no longer considered useful in sedative-hypnotic or tricyclic overdose, physostigmine is currently recommended as a diagnostic and therapeutic agent for antimuscarinic poisoning (Burns et al., 2000). Naturally available 3aS()-physostigmine is over 100 times more effective in inhibiting acetylcholinesterase and butylcholinesterase in tissue, erythrocytes, and serum in humans and animal models (Atack et al., 1989; Barak et al., 2009; Brossi, 1985, Brossi et al., 1986; Chen et al., 1992; Hill and Newkome, 1969; Petcher and Pauling, 1973; Yu et al., 1997). This stereoselectivity was recently demonstrated to depend upon asymmetric interactions within the acetylcholinesterase active center hydrophobic pocket, which is distinct from the catalytic site (Barak et al., 2009). Unexpectedly, at dosages which insignificantly inhibited acetylcholinesterase, (þ)-physostigmine protects against lethal sarin exposures and inhibits sarininduced motor endplate postjunctional damage and myopathy (Harris et al., 1990; Kawabuchi et al., 1988). This sarinprotective mechanism is apparently due to independent (þ)physostigmine antagonism at the nicotinic receptor. Physostigmine binding at nicotinic receptors is close to, but distinct from the acetylcholine binding site on the a-subunit (Pereira et al., 2002). At low doses, ()-physostigmine functions as an ineffective receptor agonist, whereas at higher doses it produces marked channel blockade (Militante et al., 2008). In addition to antidotal considerations, a more complete understanding the site-specific properties of stereoisomers of physostigmine and its carbamate analogues (e.g., at acetylcholinesterase vs. the acetylcholine receptor) aims to further drug development efforts to treat Alzheimer’s disease and myasthenia gravis. Leucovorin Leucovorin (folinic acid, 5-formyltetrahydrofolic acid) is provided as rescue therapy to patients receiving high doses of the antimetabolite antifolate methotrexate (MTX) and is used to counteract MTX toxicity (Bleyer, 1977; Flombaum and Meyers, 1999; Smith and Nelson, 2008). Endogenously produced as l-leucovorin, it had been commercially available only as the racemate until 2008, when levoleucovorin [(6S)leucovorin] received FDA approval (Spectrum Pharmaceuticals, 2008). Only the active S-form in racemic leucovorin is metabolized to reduced folates (tetrahydrofolate, 5-CH3tetrahydrofolate, 10-CHO-tetrahydrofolate, and 5,10-CH2tetrahydrofolate) (Bunni and Priest, 1991). During intravenous administration of the racemate, the active l-form (6S) conversion into 5-methyl-tetrahdrofolate is rapid, whereas the inactive isomer is slowly eliminated by renal excretion (Schilsky and Ratain, 1990). Oral bioavailability of leucovorin is poor above 40 mg and is negligible for the d-(6R)-form (Bleyer, 1989; Schilsky and Ratain, 1990). The renal tubular cell is the only cell where the inactive form is transported actively; however, under circumstances of high or frequent intravenous racemic leucovorin doses, d-leucovorin can compete with and inhibit levoleucovorin passive transmembrane transport (Bleyer, 1989). Compared with the d-isomer, levoleucovorin is 20 times more effective in inhibiting carriermediated membrane transport of methotrexate and 100-times more effective in preventing MTX growth inhibition in murine tumor cells (Sirotnak et al., 1979). As it is entirely active, levoleucovorin is prescribed at one-half of the usual racemic dose (Spectrum Pharmaceuticals, 2008). Levoleucovorin at this dose appears to provide as efficacious rescue treatment as the racemate in high dose MTX chemotherapy (Goorin et al., 1995; Jaffe et al., 1993). Glucarpidase Glucarpidase (carboxypeptidase G2, CPDG2) is undergoing evaluation as an antidote for methotrexate toxicity. This bacterially derived enzyme cleaves glutamate residues from methotrexate to render it inactive. Carboxypeptidase’s affinity for methotrexate is 10- to 15-fold higher than for leucovorin; its affinity for folate and 5-methyltetrahydrofolate (leucovorin’s active metabolite) are similar (Albrecht et al., 1978; European Medicines Agency [EMEA], 2008; Sherwood et al., 1985). CHIRAL TOXICOLOGY Active levo-(6S)-leucovorin is inactivated about 50% faster than nonphysiologic dextro-(6R)-leucovorin by glucarpidase (Hempel et al., 2005). The cleavage site is distinct from the chiral carbon. Fifteen minutes after CPDG2 administration, median leucovorin and active 5-methyltetrahydrofolate concentrations dropped by 8% and >97% (Widemann et al., 1998). Remaining leucovorin was likely in the inactive d-form (Widemann and Adamson, 2006). In healthy volunteers, glucarpidase reduced active leucovorin and activated levo-5methyltetrahydrofolate exposures by 50 and 100% despite a 2-h window between drug administration (European Medicines Agency [EMEA], 2008). Human in vivo glucarpidase activity against active leucovorin and activated levo-5-methyltetrahydrofolate has been shown to persist for at least 26 h (European Medicines Agency [EMEA], 2008). Because of this stereoselective destruction of active leucovorin and its metabolite, many protocols separate leucovorin administration from glucarpidase administration by 2–4 h. Dexrazoxane Following extravasation of anthracycline drugs, dexrazoxane [S(þ)-1,2-bis(3,5-dioxopiperazin-1-yl)propane, ICRF-187] is used to diminish tissue damage and need for surgical excision of necrosis (Mouridsen et al., 2007). Its mechanism of action appears to involve reversible inhibition of topoisomerase II and inhibition by its metabolite, an ethylenediaminetetraacetic acid analog, of free radical formation via iron removal from the iron-doxorubicin complex (Hasinoff and Aoyama, 1999; Reeves, 2007). For this reason, dexrazoxane is also used to limit anthracycline-associated cardiomyopathy (van Dalen et al., 2008). Dexrazoxane was developed due to the limited solubility of racemic razoxane (ICRF-159) (Zhang et al., 1994). In vitro, both dexrazoxane and its stereoisomer levrazoxane (ICRF-186) are equally cytotoxic and inhibitory toward DNA topoisomerase II (Hasinoff et al., 1995). However, the enzyme dihydropyrimidine amidohydrolase in liver and kidney stereoselectively catalyzes the first ringopening step in dexrazoxane versus levrazoxane, and can subsequently open the second ring only in dexrazoxane, which is required for activity (Hasinoff, 1994; Hasinoff and Aoyama, 1999) At lower doses, corresponding to those used clinically, dexrazoxane improved survival in anthracycline treated animals, and at some doses additionally demonstrated increased cardioprotection and a decrease in immune effector cells (Hasinoff and Aoyama, 1999; Zhang et al., 1994). These differences disappeared at higher doses. D-Penicillamine Penicillamine is used in the treatment of copper poisoning and Wilson’s disease and has the advantage of oral availability. D-(S)- and L-(R)-penicillamine chelate copper equally. Penicillamine is second- or third-line therapy for lead and mercury poisoning. Differential toxicity of the L- and D-forms was 15 reported as early as 1948—L-penicillamine inhibited growth and produced generalized seizures in rats (Wilson and Du Vigneaud, 1948). The L-enantiomer was also associated with impaired L-amino acid intestinal absorption (Wass and Evered, 1970), optic neuritis (Kean et al., 1991; Tu et al., 1963), nephrotic syndrome (Sternlieb, 1966) and pyridoxine antagonism (Williams, 1990). Historically, in the United Kingdom, only the D-form obtained from hydrolysis of penicillin was used, whereas U.S. manufacturers originally synthesized penicillamine from racemic valine (Walshe, 1992). Cases of thrombocytopenia and leukopenia became less prevalent when United States use of the racemic drug was discontinued (Williams, 1990). CARCINOGENICITY AND MUTAGENICITY DNA’s unique three-dimensional structure may interact with certain compounds stereospecifically (e.g., as previously detailed with cisplatin). Nucleoside analog chemotherapeutic agents (clofarabine, cytarabine, fludarabine, gemcitabine, etc.) intentionally imitate native nucleoside 2R,5R structure in order to inhibit replication. Rats treated with (S)-N#-nitrosonornicotine, a tobacco-specific carcinogenic nitrosamine, generated three to five times higher levels of metabolite-DNA adducts than with (R)-N#-nitrosonornicotine in the esophagus, a known site of tumorogenesis (Lao et al., 2007). Epoxide transformations, in particular, provide the opportunity for stereospecific carcinogenicity or mutagenicity. P450 enzymes convert the dietary and respirable dust contaminant aflatoxin B1 to aflatoxin B1-8,9-epoxide, the toxicant. Exo-aflatoxin B18,9-epoxide interacts with DNA (forming guanine adducts in particular) and is least 500-fold more potent than the endostereoisomer (Fig. 7A) (Stewart et al., 1996). Similarly, P450 enzymes convert styrene to styrene 7,8-epoxide, also present in two enantiomeric forms. The R(þ)-enantiomer is more mutagenic than the S()-enantiomer in S. typhimurium TA100 strains, with the racemic mixture intermediate between the two (Pagano et al., 1982). R(þ)-styrene 7,8-epoxide depletes glutathione to a greater extent than S-styrene oxide (Carlson et al., 2006). Benzo[a]pyrene, a polycyclic aromatic hydrocarbon implicated in tumorogenesis, is stereospecifically metabolized by cytochrome P450 to the reactive ultimate toxicant (þ)-7R,8S-dihydrodiol-9S,10R-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene, which subsequently reacts to primarily form (þ)-trans-guanine adducts (Mocquet et al., 2007). Nucleotide excision repair of the induced DNA lesions is also carried out in a stereospecific manner. 1,3-Butadiene is metabolized by P450 2E1 to butadiene monoepoxide, and subsequently undergoes epoxidation and epoxide hydrolysis to generate (R,R)- or (S,S)-butadiene diolepoxide isomers. In an in vivo bacterial expression system, the (R,R)-enantiomer induced base pair A to G mutations exclusively, whereas adducts of the (S,S)-enantiomer were exclusively A to C mutations (Carmical 16 SMITH Thalidomide FIG. 7. Examples of chiral carcinogens and teratogens. Isomers of (A) aflatoxin B1-8,9-epoxide, (B) thalidomide, and (C) retinoic acid. Thalidomide undergoes chiral inversion in humans. et al., 2000). Cis- and trans-methyl epoxycinnamates (methyl 3-phenyl-2,3-epoxy-propanoates) display opposite propensities for rat hepatic glutathione conjugation (cis- > > trans-) and Ames test mutagenicity (trans- >> cis-) (Rietveld et al., 1988). Thus, stereoisomers may produce unique mutations. REPRODUCTIVE TOXICITY Chiral factors are implicated in the actual teratogenic mechanism of several compounds. Stereospecific maternal or fetal enzymes, fetal receptors, or transplacental transport mechanisms might all contribute to toxicity. Several examples follow where stereospecific mechanisms of human teratogenicity are supported. Animal embryologic toxicity of compounds primarily considered to be environmental pollutants is contained in a following section. Thalidomide apparently works by tumor necrosis factoralpha inhibition, inhibition of angiogenesis, and other mechanisms. R(þ)-thalidomide was reported to be responsible for sedative effects (Eriksson et al., 2000; Hoglund et al., 1998), whereas S()-thalidomide and its derivatives were reported to be teratogenic (Fig. 7B) (Blaschke et al., 1979; Heger et al., 1994). It was further proposed that the thalidomide tragedy could have been avoided if the single R(þ)-enantiomer had been used. Irrespective of the fact that an earlier study in an appropriate animal model demonstrated equivalent teratogenic potential of both isomers, which was greater than the racemate (Fabro et al., 1967), chiral inversion occurs with thalidomide (Reist et al., 1998). Humans interconvert (S)- and (R)thalidomide enantiomers (Fig. 7B) rapidly with both oral and intravenous dosing (Eriksson et al., 2001). Albumin, hydroxyl ions, phosphate, and amino acids appear to mediate this effect (Reist et al., 1998). Therefore, even if single enantiomers of thalidomide were provided, the ensuing enantiomeric mixture could contribute to toxicity (Agranat et al., 2002). Similar inter-conversion has been demonstrated for thalidomide analogues (e.g., lenalidomide, EM 12, CC-4047), although certain substitutions can confer optical stability (Schmahl et al., 1988; Teo et al., 2003; U.S. Food and Drug Administration, 2005; Yamada et al., 2006). On the basis of the animal study demonstrating teratogenicity of both thalidomide isomers and evidence of human chiral inversion, exposure to either thalidomide enantiomer or unstable thalidomide derivatives during the period of sensitivity (days 20–36 after conception; Dencker and Eriksson, 1998) would risk fetal harm. Susceptibility might additionally be influenced by genetic polymorphisms which alter thalidomide metabolism (Ando et al., 2002) in the maternal/fetal unit. Retinoic Acids Following a 1979 landmark report, oral isotretinoin revolutionized the treatment of recalcitrant cystic and conglobate acne (Peck et al., 1979). Alitretinoin (9-cis retinoic acid for topical treatment of cutaneous Kaposi’s sarcoma lesions) and isotretinoin (13-cis retinoic acid) are isomers of tretinoin (all-trans retinoic acid) (Fig. 7C). Teratogenic effects are presumed to be mediated in part via transformation of alitretinoin and isotretinoin to all-trans retinoic acid (Adams, 1993). Variations in teratogenicity among the isomers also likely arise secondary to species-specific, stereoselective tissue transport (Collins and Mao, 1999). Cis-isomerization of the retinoic acid side chain reduces the teratogenicity (Collins and Mao, 1999). However, transplacental exposures of therapeutic doses of isotretinoin were sufficient to produce well described, severe teratogenic effects—including spontaneous abortion; craniofacial, cardiac, thymic, and CNS malformations; motor and sensory deficiencies; and sex-susceptible cognitive impairment (Lammer et al., 1985; McCaffery et al., 2003). Persistent 17 CHIRAL TOXICOLOGY fetal exposures led to the creation of one of the most stringent risk management prescribing programs in the United States (iPLEDGE) (Honein et al., 2007). Excess exogenous retinoids disrupt the precise retinoic acid concentrations at specific stages of embryonic development which are required for induction of anterior-posterior development of the brain, dorsal-ventral development of the spinal cord, and some sexual dimorphic traits (McCaffery et al., 2003). Structure does confer receptor specificity—retinoic acid receptors (RARs) bind both all-trans and 9-cis retinoic acid, whereas only 9-cis retinoic acid binds to retinoid X receptors, which partner with RARs, vitamin D receptors, thyroid hormone receptors, peroxisome proliferator–activated receptors, and others (Germain et al., 2006a, b). The low (1–2%) percutaneous absorption of topical 0.05% tretinoin does not significantly increase systemic retinoid plasma concentrations above the range of natural endogenous levels (Thielitz and Gollnick, 2008). Although isolated case reports suggested a link between topical tretinoin exposure and fetal congenital abnormalities, several studies (215:430, 106:389, and 94:133 case-controls), failed to detect any effect (Jick et al., 1993; Loureiro et al., 2005; Shapiro et al., 1997). The U.S. FDA pregnancy classification for topical tretinoin (up to 0.1%) (category C), reflects this decreased perceived risk compared with the oral formulation (category X). While not U.S. FDA approved, systemic absorption of topical isotretinoin gel (up to 0.1%) is negligible despite repeated application (Thielitz and Gollnick, 2008). Although formal studies are lacking, daily application of topical alitretinoin gel (0.1%) (pregnancy category D) for up to 60 weeks did not yield detectable 9-cis-retinoic acid metabolites or 9-cis-retinoic acid plasma concentrations above those in untreated healthy volunteers (Eisai, Inc., 2007). Factors increasing dermal systemic absorption could alter teratogenic potential of the topical formulations. Valproic Acid Fetal VPA syndrome produces a consistent facial phenotype, systemic and orthopedic involvement, CNS dysfunction, and altered physical growth and cognitive development. It is thought to occur due to exposure at weeks 20–24 as the neural tube is closing. VPA and its S-derivatives appear to inhibit histone deacetylase, which leads to increased unfolding of chromatin and increased gene expression (Eikel et al., 2006). In particular, pattern-forming, homeobox A1 (Hoxa1) mRNA is produced in excess and outside of the normal developmental periods following VPA and 4-yn-VPA exposure (Stodgell et al., 2006). The VPA metabolite R(þ)- and S()-enantiomers of 2-n-propyl-4-pentenoic acid (4-en-VPA) and 2-n-propyl-4pentynoic acid (4-yn-VPA) significantly differ in teratogenicity (Nau et al., 1991). Almost four times more teratogenic than VPA, (S)-4-yn-VPA is 7.5 times more teratogenic than the (R)isomer, and 1.9 times more teratogenic than the racemate, despite similar neurotoxicity (Hauck and Nau, 1992). Whole embryo cultures confirmed that (S)-4-yn-VPA produced dose- dependent dysmorphogenesis and embryo death in contrast to (R)-4-yn-VPA (Andrews et al., 1995). ENVIRONMENTAL TOXICOLOGY Many environmental contaminants are chiral, including organophosphorus compounds, organochlorines, pyrethroids, PCBs, polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), fipronil, and pharmaceutical contaminants. Degradation of these compounds, as well as compound bioaccumulation, persistence, and toxicity often show chiral dependence. Organophosphorus Compounds If the three substituent atoms are different, then the phosphorous atom is a chiral center (Fig. 8A). In geographically distinct areas, enantioselective degradation of organophosphorus compounds occurs (Lewis et al., 1999). FIG. 8. (A) Enantiomers of substituted organophosphorus compounds (X 6¼ Y 6¼ Z). (B) Chiral centers of the pyrethroids permethrin, cypermethrin, cyfluthrin, and bifenthrin—the asterix (*) denotes chiral atoms. If the R1 and R2 substituents differ (e.g., in bifenthrin), then additional E- and Z-isomers are possible. If R3 contains a nonhydrogen substituent group such as CN, an alternative chiral carbon (#) is present. (C) PCB 139 (2,2#,3,4,4#,6hexachlorobiphenyl)—one of several PCBs with stable atropisomers which have stereospecific effects in vivo. PCB atropisomers have generally been identified on the basis of optical activity, as assignment of absolute configuration is difficult. 18 SMITH Some bacteria contain phosphotriesterases capable of catalyzing the cleavage of the phosphate-oxygen bond and degrading the toxic organophosphate esters. Differences in geography, temperature, and deforestation can cause these bacteria to switch which enantiomer is preferentially degraded (Lewis et al., 1999; Wong, 2006). As might be anticipated from the previous discussion of acetylcholinesterase stereoselectivity in relation to physostigmine, significant enantiomer- and speciesspecific acetylcholinesterase susceptibility has been demonstrated for several organophosphorus insecticides. This occurs in such diverse species as Daphnia magna (water flea, the standard model of environmental toxicity), the electric eel, and humans (Nillos et al., 2007; Zhou et al., 2007). Therefore, chirality is an important aspect in ascertaining the environmental impact of organophosphorus compounds. The stereospecificity seen in organophosphorus insecticides extends to chemical warfare nerve agents. Certain enantiomers are significantly more toxic, and show prolonged in vivo persistence due to variable stereospecific protein binding (Yeung et al., 2008). Variable acetylcholinesterase inhibition has been demonstrated for tabun, sarin, soman (with two chiral centers), and VX (Benschop and De Jong, 1988). These stereospecific differences might be anticipated given selectivity of the active site previously described for carbamates. Pyrethroids The pyrethroids, sodium channel opener insecticides derived from chrysanthemum flowers, have at least 2 chiral centers. Those with cyano-substitution at the a-carbon may have an additional chiral center (Fig. 8B). This generates 4 or 8 possible stereoisomers. Pyrethroids may isomerize slowly at the asymmetric a-carbon atom in polar solvents and in the presence of light (Wong, 2006). The trans-diastereomers of b-cypermethrin and b-cyfluthrin are selectively degraded in alkaline soils (Li et al., 2008). Pesticide-degrading bacteria selectively metabolize cis-bifenthrin and permethrin (Liu et al., 2005a). In geographically distinct separate soil samples, enantioselective microbial-mediated degradation was frequently observed for cis-bifenthrin, permethrin, and cyfluthrin; the rate was dependent upon variations in the microbial content, soil type, pH, and environmental conditions (Qin et al., 2006). Stereospecific toxicity has been observed for the pyrethroids. The (1R)-isomers of cycloprothrin were significantly more larvacidal than the (1S) enantiomers (Jiang et al., 2008). The two enantiomers of cis-bifenthrin and permethrin with the (1R) configuration, that is, (1R)-cis and (1R)-trans, were substantially more toxic to ‘‘bystander’’ water fleas (Ceriodaphnia dubia and Daphnia magna, an important food sources for many larger aquatic organisms) (Liu et al., 2005b). Only two of the eight cypermethrin and cyfluthrin enantiomers, (1R)-cis-alpha-S and 1R-trans-alpha-S, had significant activity, with 10–100 times more toxicity. Levolambda-cyhalothrin was over 162 times more acutely toxic than dextro-lambda-cyhalothrin to zebrafish (Xu et al., 2008a). It was also more embryocidal and teratogenic. Cispermethrin exposed mice had significant reproductive toxicity including reduced epididymal sperm counts and motility, and a decrease in testes and plasma testosterone levels not seen with the trans-isomer, which appeared to have faster metabolism (Zhang et al., 2008). Preliminary data also suggests that (1S)-cis-bifenthrin is a much more significant estrogen disruptor than the (1R)-enantiomer. (Wang et al., 2007). Polychlorinated Biphenyls, Dioxins, Dibenzofurans, and Organochlorines PCBs were used as coolants and lubricants in transformers, capacitors, and other electrical equipment. Although U.S. domestic production was banned in 1977, they remain present in numerous hazardous waste sites. In humans, exposure to PCBs has been linked to chloracne, ocular and pulmonary symptoms in adults, neurobehavioral and immunological changes in children, teratogenicity, and hepatic carcinoma (Aoki, 2001). Additional thymic, CNS, and reproductive toxicities exist in animals (Aoki, 2001;Tanabe, 1988). The 209 theoretical PCB cogeners are named and numbered sequentially according to a variety of sources: IUPAC nomenclature, Chemical Abstracts Service number, and the original Ballschmiter and Zell enumeration as modified by several authors (Mills et al., 2007). Seventy-eight PCBs demonstrate axis chirality (existing as rotational nonsuperimposable isomers); 19 are stable atropisomers in biota (Fig. 8C) (Haglund, 1996). Several PCB atropisomers display enantiospecific P450 enzyme interactions (detailed in Haglund, 1996; Kania-Korwel et al., 2008). PCBs also demonstrate speciesspecific accumulation—for example, in pelicans, seals, and polar bears (Karasek et al., 2007; Wiberg et al., 1998). Furthermore, metabolite fractions are different from the original food-source, indicating enantio-selective formation, metabolism, transport, or clearance. Moreover, tissue-specific and organ-specific enantiomer-selective retention has been shown in rats. Compared with adipose and liver tissue, rat lung tissues had reversed enantiomer preferences for PCB 149 metabolites (Larsson et al., 2002). Enrichment of specific PCB 95, PCB 149, and PCB 132 isomers is reported in human liver samples (Chu et al., 2003). The concentration of at least five relevant PCB congeners are enriched in milk and dairy products from cows, goats, and ewes in a dairy-product and species-specific manner (Bordajandi and Gonzalez, 2008). This is presumed to occur through species-dependent metabolism as well as further transformations by microorganisms during the fermentation and ripening process. Lastly, levo-PCB 136 was recently shown to enantioselectively enhance ryanodine binding at ryanodine type 1 and 2 receptors and increase calcium flux, raising the possibility that chiral PCBs may have additional toxicological mechanisms (Pessah et al., 2009). CHIRAL TOXICOLOGY In addition to the multiple PCB isomers, 456 of 837 possible methylsulfonyl-PCB-derivatives are chiral (Nezel et al., 1997). The mercapturic acid pathway forms methyl sulfone metabolic products of PCBs (MeSO2-CB) (Karasek et al., 2007). Microbial reductive dechlorination—the major degradation mechanism for PCBs under anaerobic conditions—occurs in an enantiospecific manner for certain PCBs (e.g., PCB 91 and PCB 95) (Pakdeesusuk et al., 2003). Examining the specific isomers thus helps elucidate the contribution of biological activity (natural attenuation) versus chemical, distribution, and transport processes in contaminated ecosystems (Nezel et al., 1997). Although atmospheric PCB contributions are racemic, certain PCBs are nonracemic in water, suspended particulate matter, sediments and phytoplankton (which absorb PCBs by passive diffusion and lack the ability to metabolize them [Asher et al., 2007]). As discharge of water from a previously contaminated upstream source increased, the enantiomer fraction of PCBs was obtained down-stream diverged from the racemate, leading to the identification of transport of contaminated sediment as the major PCB source. Thus, the concept of chiral fingerprinting or ‘‘chiral signatures’’ of PCBs can now being applied to differentiate among PCBs source contamination from upstream river-water, storm-water runoff, sewer overflows, and atmospheric deposition from urbangenerated PCBs (Asher et al., 2007). As early as 1969 it was noted that the stereoisomers of cyclodiene insecticides could produce different toxicity. Mice fed endrin at 5 ppm suffered a 33% mortality, whereas dieldrin (its stereoisomer) was no different than control (Good and Ware, 1969). Both compounds reduced the litter size of offspring. Similar to the multiple PCB congeners, PCDD molecules can exist in 75 possible planar isomers. Isomer toxicity varies greatly by factors of 1000 to 10,000 (Boening, 1998). Isomeric analysis of agricultural soils permits source determination of PCDDs and PCDFs (municipal incinerators versus agrochemical impurities) (Xu et al., 2008b). Depending on soil type and location, preferential degradation of either enantiomer of o,p#-DDT may occur (Aigner et al., 1998; Li et al., 2006). The ()-o,p#-DDT enantiomer is an active estrogen mimic at the human estrogen receptor, whereas (þ)-o,p#-DDT was negligible (Hoekstra et al., 2001). Thus, the environmental impact of organochlorines must be approached from a geo-local, species-specific, and organ-specific perspective. Fipronil Fipronil, a racemic phenylpyrazole pesticide, is a noncompetitive GABA receptor antagonist available in the United States since 1996. Humans exposed to fipronil may have headache, nausea, and seizures, and prolonged GABAA receptor blockade has been demonstrated in mammalian brains (Li and Akk, 2008). Fipronil is also used in commercial pet products. Fipronil’s environmental fate is sediment-dependent: preferential transformation of S(þ)-fipronil occurs in anoxic 19 sulfidogenic sediment, whereas preferential transformation of R()-enantiomer in methanogenic anoxic sediment (Jones et al., 2007). In a geographically disparate area, under flooded (anaerobic) conditions, (S)-fipronil was preferentially degraded in methanogenic soil samples, whereas no selectivity was noted under aerobic conditions, a difference attributed to alternative pH, temperature, soil water content, organic matter content or microbial populations (Tan et al., 2008). Determination of the persistent enantiomer is important to assess environmental risk. Although highly selective to insect nerve cells, ‘‘aquatic bystanders’’ are susceptible to species-specific enantiomer toxicity of fipronil. C. dubia is selective affected by the (S)enantiomer (Wilson et al., 2008). Similarly, crayfish are significantly more sensitive to the (S)-enantiomer, whereas larval grass shrimp are significantly more sensitive to the (R)enantiomer (Overmyer et al., 2007). Highlighting the economic importance of understanding these complex enantiomer relationships, the introduction of rice seed treated with fipronil produced major losses in Louisiana crayfish harvests, and degradation products were found to persist for several years (Bedient et al., 2005). Fipronil is rapidly biotransformed by the rainbow trout, which selectively transform the (S)-enantiomer. The sulfone metabolite has a thrice-greater half-life and thus may bioaccumulate (Konwick et al., 2006). Future environmental assessment of fipronil will have to account for local conditions, enantio-selective food-source toxicity, and bioaccumulation. Pharmaceutical Contaminants Pharmaceuticals may contaminate the environment following unchanged parent compound elimination from humans or animals or active disposal in solid or liquid waste streams. Pharmaceutical residues persist nearly year round in some major waterways (Comeau et al., 2008; Sacher et al., 2008). However, relatively little work has been done on chiral aspects of these xenobiotics or their significance. The NSAIDs and ibuprofen in particular can be found in many rivers and lakes; the active (S)-enantiomer was preferentially degraded in lake water (Buser et al., 1999). Nonpharmacologically active R()ibuprofen was degraded more rapidly by microorganisms in biofilm reactors than the pharmacologically active isomer (Winkler et al., 2001). In aquatic toxicity studies, growth and feeding of P. promelas (fathead minnow) were more adversely affected by S-fluoxetine than R-fluoxetine, whereas water fleas did not have stereospecific susceptibility (Stanley et al., 2007). The enantiomeric fraction of R(þ)-propranolol decreases following wastewater treatment, and thus water samples which contained racemic mixtures of propranolol confirmed suspected discharges of untreated sewage (Fono and Sedlak, 2005). Similar studies have examined atenolol and metoprolol isomers of wastewater effluent (Nikolai et al., 2006). (R)-propranolol has greater effects on reproduction than either the racemate or the (S)-isomer at the highest tested dose in water fleas, whereas growth was affected more by (S)-propranolol in P. promelas, 20 SMITH similar to mammals (Stanley et al., 2006). Residues of 4methylbenzylidene camphor, an organic ultraviolet filter used in personal care sunscreens products, could be modified by enantioselective biodegradation and in lakes and in fish (Buser et al., 2005). FORENSIC TOXICOLOGY In forensics, chiral principles can be applied to both to drug/ product seizures and to biological samples. It has been estimated that over half of illicit compounds possess at least one chiral center (Mile, 2005). Truxilline, a tropine alkaloid, is present as 11 stereoisomers in the coca leaf. Resolution of these and other stereoisomers of cocaine-associated impurities can provide a manufacturing fingerprint for law-enforcement intelligence and strategic purposes (Tagliaro et al., 2007). Illicitly produced heroin is commonly ‘‘cut’’ with sugars; thus, the chiral analysis of these in addition to other excipients (phenacetin, caffeine, etc.) may provide important information for forensic purposes (Lurie, 1998; United Nations Office on Drugs and Crime, 2005). Chiral analysis can be applied to other drug seizures. For example, presumed methamphetamine seizures might demonstrate levo-ephedrine and dextromethamphetamine, which would be consistent with levo-ephedrine as the precursor material. Such analysis can be extended to other phenylethylamine derivatives such as ‘‘ecstasy’’ (commonly 3,4-methylenedioxymethamphetamine) and N,N-dimethylamphetamine to provide clues to precursor materials and synthesis pathways (Lee et al., 2007; Tagliaro and Bortolotti, 2008). Due to the very different U.S. DEA scheduling of racemic methorphan (CII, not clinically available in the United States), levomethorphan (CII, not clinically available in the United States) and dextromethorphan (unscheduled), forensic determination of methorphan isomers seizures must be performed in order to support illegality (Lurie and Cox, 2005). Chiral principles must be applied to correctly interpret the significance of ‘‘positive’’ biological samples from employment or post-mortem drug testing. As an example, certain amphetamine or methamphetamine enantiomer compositions may or may not be appropriate following a reported history of ingestion (Fig. 9). Methamphetamine is metabolized unidirectionally to amphetamine (and 4-hydroxymethamphetamine) (Jirovsky et al., 1998). d-Methamphetamine is metabolized more rapidly than the l-enantiomer; thus, the enantiomer ratio will change over time (Cody, 2002). Pharmacodynamic differences include induction of higher systolic blood pressures and more prolonged and psychologically desirable effects with the d-isomer (Mendelson et al., 2006). Finding methamphetamine would undermine a claim of medical legitimacy in xenobiotics composed of or directly metabolized to amphetamine—for example, one would expect to find a mixture of d-and l-amphetamine, but not methamphetamine in individuals consuming Adderall [d,l-amphetamine salts (3:1), DEA schedule II] (Barr Laboratories, 2007). Similarly, Dexedrine (dextroamphetamine sulfate, DEA schedule II) should only result in serum concentrations of d-amphetamine, and only d-amphetamine should be detected following ingestion of Vyvanse (lisdexamfetamine dimesylate, DEA schedule II), a dextroamphetamine pro-drug (Fig. 9). Vicks Vapor Inhaler (unscheduled) contains 50 mg of l-methamphetamine (per inhaler). Forensic specimens should contain only the l-isomer and the l-amphetamine metabolite. Conversely, Desoxyn (d-(S)-methamphetamine hydrochloride, DEA schedule II)— prescribed for attention deficit disorder with hyperactivity and exogenous obesity—should yield solely the d-isomers. Didrex (d-benzphetamine, DEA schedule III) is an anti-obesity drug marketed in the United States. Metabolism to d-methamphetamine and separate metabolism to d-amphetamine result in much higher d-amphetamine to d-methamphetamine ratios (mean ¼ 2.4) than normally seen in methamphetamine abuse (without generation of l-isomers) (Cody, 2002). Eldepryl (selegiline hydrochloride, unscheduled), used in the treatment of Parkinson’s disease, is manufactured only as the l-isomer. A certain percentage is metabolized to (only) l-methamphetamine and l-amphetamine. Clobenzorex is an anorexic drug now illegal in US, but abused in professional sports. It is available abroad (as Asenlix, Dinintel, and Finedal) as part of unapproved diet regimens utilized by U.S. residents (U.S. Food and Drug Administration, 1987). Approximately 5% is converted to racemic amphetamine (Baden et al., 1999). Fenproporex (DEA schedule IV) is appetite suppressant used outside U.S. FDA action occurred in 2006 because the ‘‘Brazilian Diet Pill’’ contained this compound and resulted in workplace drug testing ‘‘failures’’ (U.S. Food and Drug Administration, 2006). Roughly 25–35% is converted to racemic amphetamine. Methamphetamine and amphetamine metabolites of mefenorex (CIV), another anorectic marketed primarily in Europe, may persist beyond the parent compound (Engel et al., 1986; Musshoff, 2000). Gewodin is an over the counter antipyretic/analgesic multi-ingredient product available in Germany, Taiwan, and elsewhere, containing acetaminophen, propyphenazone (isopropylphenazone), caffeine, and famprofazone (Musshoff and Kraemer, 1998). Stereoselective famprofazone metabolism leads to significantly more l-methamphetamine production than l-amphetamine or the d-isomers (Rodriguez et al., 2004). With wide interindividual variability, the calcium channel antagonist and anti-anginal prenylamine (unscheduled) metabolizes to significantly more d-metabolites (and d-amphetamine) than l-amphetamine (Kraemer et al., 2003; Liu and Liu, 2002). Amphecloral (INN: amfecloral) is a phenethylamine derivative which was patented in 1960 as an amphetamine pro-drug with prolonged duration of action (Cavallito, 1960; Kolliker and Oehme, 2004). Metabolism yields chloral hydrate plus d-amphetamine or d,l-amphetamine, depending on the method of preparation (Fig. 9). Other compounds which metabolize to methamphetamine or amphetamine include amphetaminil (unscheduled), CHIRAL TOXICOLOGY 21 FIG. 9. Pharmaceuticals that contain or are metabolized to amphetamine isomers and methamphetamine isomers. Solid lines indicate that no metabolism is required; dotted lines indicate that metabolism occurs. Amphecloral, clobenzorex, fenproporex, and prenylamine are metabolized to both d-(S)- and l-(R)amphetamine. dimethylamphetamine (CI), ethylamphetamine (CI), fencamine (unscheduled), fenethylline (CI), furfenorex (unscheduled), and mesocarb (unscheduled) (Cody, 2002; Liu and Liu, 2002; Musshoff, 2000). Thus, a detailed history of reported xenobiotic ingestion and appropriate isomer analysis may support or refute a claim of legitimate pharmaceutical ingestion or aid in the determination of cause of death. Chiral analysis has been applied to screen athletes for exogenous administration of androgens since 1983 (Bowers, 2008). The endogenous steroid profile of testosterone (T) to its enantiomer epitestosterone (E)—the urinary T/E ratio—occurs in populations in two modal distributions in both sexes at about 0.1 and 1.0 (Bowers, 2008). Rare cases of physiologically high T/E ratios (between 6 and 12) and low T/E ratios (due to deletion polymorphism in the uridine diphospho-glucuronosyl transferase 2B17 gene) are reported (Dehennin, 1994; Schulze et al., 2008). According to the rules of the World Anti-Doping Agency, an E/T glucuronide ratio equal to or exceeding 4 to 1 is an atypical result suggestive of exogenous androgenic steroid administration and requires further investigation (World 22 SMITH Anti-Doping Agency, 2007). T/E ratios can be coupled with evaluation of 13C/12C ratios (as pharmaceutically produced anabolic steroids exhibit a depleted ratio on account of their plant origin) to provide additional evidence of a doping offense (Piper et al., 2008). CONCLUSION Chiral considerations are relevant to diverse aspects of toxicology and pharmacology. The additional complexity permits a more complete and precise understanding of toxicological pathophysiology. Evaluation of chiral compounds must take into account three-dimensional structureactivity relationships, which may take on varied importance at different receptor types. Local conditions species and tissue differences and population polymorphisms may additionally influence enantiomer ratios and xenobiotic effects. ACKNOWLEDGMENTS This manuscript was developed from a presentation at the American College of Medical Toxicology Spring Conference, San Diego, CA, USA, March 2008. The author is indebted to Lewis S. Nelson, MD, FACEP, FACMT, and Mary Ann Howland, PharmD, DABAT, FAACT for their insightful suggestions and manuscript review. REFERENCES ¨ ber das verhalten des blutdruckes Abderhalden, E., and Mu¨ller, F. (1908). U nach intraveno¨ser Einfu¨rung von l-, d- und dl-Suprarenin. Z. Physiol. Chem. 58, 185–189. Adams, J. (1993). Structure-activity and dose-response relationships in the neural and behavioral teratogenesis of retinoids. Neurotoxicol. Teratol. 15, 193–202. Agranat, I., Caner, H., and Caldwell, J. (2002). Putting chirality to work: The strategy of chiral switches. Nat. Rev. Drug Discov. 1, 753–768. Ahuja, S., Crocker, E., Eilers, M., Hornak, V., Hirshfeld, A., Ziliox, M., Syrett, N., Reeves, P. J., Khorana, H. G., Sheves, M., et al. (2009). Location of the retinal chromophore in the activated state of rhodopsin. J. Biol. Chem.doi:10.1074/jbc.M805725200. Aigner, E. J., Leone, A. D., and Falconer, R. L. (1998). Concentrations and enantiomeric ratios of organochlorine pesticides in soils from the U.S. corn belt. Environ. Sci. Technol. 32, 1162–1168. Albrecht, A. M., Boldizsar, E., and Hutchison, D. J. (1978). Carboxypeptidase displaying differential velocity in hydrolysis of methotrexate, 5-methyltetrahydrofolic acid, and leucovorin. J. Bacteriol. 134, 506–513. Ali, I., Gupta, V. K., Aboul-Enein, H. Y., Singh, P., and Sharma, B. (2007). Role of racemization in optically active drugs development. Chirality 19, 453–463. Anderson, J. L., Horne, B. D., Stevens, S. M., Grove, A. S., Barton, S., Nicholas, Z. P., Kahn, S. F., May, H. T., Samuelson, K. M., Muhlestein, J. B., et al. (2007). Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116, 2563–2570. Ando, Y., Price, D. K., Dahut, W. L., Cox, M. C., Reed, E., and Figg, W. D. (2002). Pharmacogenetic associations of CYP2C19 genotype with in vivo metabolisms and pharmacological effects of thalidomide. Cancer. Biol. Ther. 1, 669–673. Andrews, J. E., Ebron-McCoy, M., Bojic, U., Nau, H., and Kavlock, R. J. (1995). Validation of an in vitro teratology system using chiral substances: Stereoselective teratogenicity of 4-yn-valproic acid in cultured mouse embryos. Toxicol. Appl. Pharmacol. 132, 310–316. Anonymous (1997). Fenfluramine and dexfenfluramine withdrawn from market. Am. J. Health. Syst. Pharm. 54, 2260–2269. Anonymous (2002). Escitalopram (lexapro) for depression. Med. Lett. Drugs Ther. 44, 83–84. Anonymous (2006). A levalbuterol metered-dose inhaler (Xopenex HFA) for asthma. Med. Lett. Drugs Ther. 48, 21– 22; 24 Aoki, Y. (2001). Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—What we have learned from Yusho disease. Environ. Res. 86, 2–11. Aps, C., and Reynolds, F. (1978). An intradermal study of the local anaesthetic and vascular effects of the isomers of bupivacaine. Br. J. Clin. Pharmacol. 6, 63–68. Arago, F. (1811). Me´moire sur une modification remarquable qu’e´prouvent les rayons lumineux dans leur passage a` travers certains corps diaphanes, et sur quelques autres nouveaux phe´nome`nes d’optique [On an interesting effect shown by light rays on their passage through certain transparent materials and some other new optical phenomena]. Me´m. Classe Sci. Math. Phys. Inst. Impe´rial France. 1, 93–134. Arancio, O., Bonadonna, G., Calvani, M., Giovene, P., Tomelleri, G., and De Grandis, D. (1989). Transitory L-carnitine depletion in rat skeletal muscle by D-carnitine. Pharmacol. Res. 21, 163–168. Asher, B. J., Wong, C. S., and Rodenburg, L. A. (2007). Chiral source apportionment of polychlorinated biphenyls to the Hudson River estuary atmosphere and food web. Environ. Sci. Technol. 41, 6163–6169. Atack, J. R., Yu, Q. S., Soncrant, T. T., Brossi, A., and Rapoport, S. I. (1989). Comparative inhibitory effects of various physostigmine analogs against acetyl- and butyrylcholinesterases. J. Pharmacol. Exp. Ther. 249, 194–202. Au, N., and Rettie, A. E. (2008). Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab. Rev. 40, 355–375. Augustijns, P., and Verbeke, N. (1993). Stereoselective pharmacokinetic properties of chloroquine and de-ethyl-chloroquine in humans. Clin. Pharmacokinet. 24, 259–269. Ayala, C. A. (1995). Stimulation of choline acetyl transferase activity by l- and d-carnitine in brain areas of neonate rats. J. Neurosci. Res. 41, 403–408. Baden, K. L., Valtier, S., and Cody, J. T. (1999). Metabolic production of amphetamine following multidose administration of clobenzorex. J. Anal. Toxicol. 23, 511–517. Baker, G. B., and Prior, T. I. (2002). Stereochemistry and drug efficacy and development: Relevance of chirality to antidepressant and antipsychotic drugs. Ann. Med. 34, 537–543. Barak, D., Ordentlich, A., Stein, D., Yu, Q. S., Greig, N. H., and Shafferman, A. (2009). Accommodation of physostigmine and its analogues by acetylcholinesterase is dominated by hydrophobic interactions. Biochem. J. 417, 213–222. Barr Laboratories, Inc. (2007). ADDERALLÒ Prescribing Information. Barr Laboratories, Inc., Pomona, NY. Bazzato, G., Coli, U., Landini, S., Mezzina, C., and Ciman, M. (1981). Myasthenia-like syndrome after D,L- but not L-carnitine. Lancet 1, 1209. Bedient, P. B., Horsak, R. D., Schlenk, D., Hovinga, R. M., and Pierson, J. D. (2005). Environmental impact of fipronil to the Louisiana Crawfish Industry. Environ. Forensics 6, 289. Benschop, H. P., and De Jong, L. P. A. (1988). Nerve agent stereoisomers: Analysis, isolation and toxicology. Acc. Chem. Res. 21, 368–374. CHIRAL TOXICOLOGY Biot, J. B. (1812a). Me´moire sur les rotations que certains substances impriment aux axes de polarisation des rayons lumineux [The rotation of the axes of polarization of light by certain substances]. Me´m. Classe Sci. Math. Phys. Inst. Impe´rial France II, 41–136. Biot, J. B. (1812b). Me´moire sur un nouveau genre d’oscillation que les mole´cules de la lumie`re e´prouvement en traversant certains cristeaux [On a new type of oscillation exhibited by light travelling through certain crystals]. Me´m. Classe Sci. Math. Phys. Inst. Impe´rial France1 II, 1–372. Blaschke, G., Kraft, H. P., Fickentscher, K., and Kohler, F. (1979). Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneimittelforschung 29, 1640–1642. Blazer, S., Khankin, E., Segev, Y., Ofir, R., Yalon-Hacohen, M., Kra-Oz, Z., Gottfried, Y., Larisch, S., and Skorecki, K. L. (2002). High glucose-induced replicative senescence: Point of no return and effect of telomerase. Biochem. Biophys. Res. Commun. 296, 93–101. Bleyer, W. A. (1989). New vistas for leucovorin in cancer chemotherapy. Cancer 63, 995–1007. Bleyer, W. A. (1977). Clinical pharmacology of intrathecal methotrexate. II. An improved dosage regimen derived from age-related pharmacokinetics. Cancer Treat. Rep. 61, 1419–1425. Boening, D. W. (1998). Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to several ecological receptor groups: A short review. Ecotoxicol. Environ. Saf. 39, 155–163. Bordajandi, L. R., and Gonzalez, M. J. (2008). Enantiomeric fraction of selected chiral polychlorinated biphenyls in cow, goat, and ewe milk and dairy products by heart-cut multidimensional gas chromatography: First results. J. Dairy Sci. 91, 483–489. Boudvillain, M., Dalbies, R., Aussourd, C., and Leng, M. (1995). Intrastrand cross-links are not formed in the reaction between transplatin and native DNA: Relation with the clinical inefficiency of transplatin. Nucleic Acids Res. 23, 2381–2388. Boulton, D. W., and Fawcett, J. P. (1997). Pharmacokinetics and pharmacodynamics of single oral doses of albuterol and its enantiomers in humans. Clin. Pharmacol. Ther. 62, 138–144. Bowers, L. D. (2008). Testosterone doping: Dealing with genetic differences in metabolism and excretion. J. Clin. Endocrinol. Metab. 93, 2469–2471. Brocks, D. R. (2006). Drug disposition in three dimensions: An update on stereoselectivity in pharmacokinetics. Biopharm. Drug Dispos. 27, 387–406. Brossi, A. (1985). Further explorations of unnatural alkaloids. J. Nat. Prod. 48, 878–893. 23 Cahn, R. S., Ingold, C., and Prelog, V. (1966). Specification of molecular chirality. Angewandte Chemie International Edition in English 5, 385–415. Cahn, R. S., Ingold, C., and Prelog, V. (1956). The specification of asymmetric configuration in organic chemistry. Experientia 12, 81–94. Caner, H., Groner, E., Levy, L., and Agranat, I. (2004). Trends in the development of chiral drugs. Drug Discov. Today 9, 105–110. Carlson, G. P., Turner, M., and Mantick, N. A. (2006). Effects of styrene and styrene oxide on glutathione-related antioxidant enzymes. Toxicology 227, 217–226. Carmical, J. R., Nechev, L. V., Harris, C. M., Harris, T. M., and Lloyd, R. S. (2000). Mutagenic potential of adenine N(6) adducts of monoepoxide and diolepoxide derivatives of butadiene. Environ. Mol. Mutagen. 35, 48–56. Cavallito, C. J. (1960). N-[2-(1-phenyl-propyl)]-2,2,2-trichloroethylidenimine. U.S. Patent Office patent number 2,923,661. patented February 2, 1960. Chen, Y. L., Nielsen, J., Hedberg, K., Dunaiskis, A., Jones, S., Russo, L., Johnson, J., Ives, J., and Liston, D. (1992). Syntheses, resolution, and structure-activity relationships of potent acetylcholinesterase inhibitors: 8-Carbaphysostigmine analogues. J. Med. Chem. 35, 1429–1434. Chu, S., Covaci, A., and Schepens, P. (2003). Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 93, 167–176. Claessens, Y. E., Cariou, A., Monchi, M., Soufir, L., Azoulay, E., Rouges, P., Goldgran-Toledano, D., Branche, F., and Dhainaut, J. F. (2003). Detecting life-threatening lactic acidosis related to nucleoside-analog treatment of human immunodeficiency virus-infected patients, and treatment with L-carnitine. Crit. Care Med. 31, 1042–1047. Clair, F., Caillat, S., Soufir, J. C., Lafforgue, B., Drueke, T., and Said, G. (1984). Myasthenic syndrome induced by D,L-carnitine in a chronic hemodialysis patient. Presse Med. 13, 1154–1155. Clarkson, T. W., Vyas, J. B., and Ballatori, N. (2007). Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 50, 757–764. Cody, J. T. (2002). Precursor medications as a source of methamphetamine and/or amphetamine positive drug testing results. J. Occup. Environ. Med. 44, 435–450. Collins, M. D., and Mao, G. E. (1999). Teratology of retinoids. Annu. Rev. Pharmacol. Toxicol. 39, 399–430. Comeau, F., Surette, C., Brun, G. L., and Losier, R. (2008). The occurrence of acidic drugs and caffeine in sewage effluents and receiving waters from three coastal watersheds in Atlantic Canada. Sci. Total Environ. 396, 132–146. Brossi, A., Schonenberger, B., Clark, O. E., and Ray, R. (1986). Inhibition of acetylcholinesterase from electric eel by (-)-and (þ)-physostigmine and related compounds. FEBS Lett. 201, 190–192. Corcoran, G. B., and Wong, B. K. (1986). Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: Studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 238, 54–61. Buhler, I., Walter, R., and Reinhart, W. H. (2001). Influence of D- and Lglucose on erythrocytes and blood viscosity. Eur. J. Clin. Invest. 31, 79–85. Covyeou, J. A., and Jackson, C. W. (2007). Hyponatremia associated with escitalopram. N. Engl. J. Med. 356, 94–95. Bunni, M. A., and Priest, D. G. (1991). Human red blood cell-mediated metabolism of leucovorin [(R,S)5-formyltetrahydrofolate. Arch. Biochem. Biophys. 286, 633–637. Crettol, S., Deglon, J. J., Besson, J., Croquette-Krokkar, M., Gothuey, I., Hammig, R., Monnat, M., Huttemann, H., Baumann, P., and Eap, C. B. (2005). Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin. Pharmacol. Ther. 78, 593–604. Burns, M. J., Linden, C. H., Graudins, A., Brown, R. M., and Fletcher, K. E. (2000). A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann. Emerg. Med. 35, 374–381. Busch, E. M., Gorgels, T. G., Roberts, J. E., and van Norren, D. (1999). The effects of two stereoisomers of N-acetylcysteine on photochemical damage by UVA and blue light in rat retina. Photochem. Photobiol. 70, 353–358. Buser, H. R., Muller, M. D., Balmer, M. E., Poiger, T., and Buerge, I. J. (2005). Stereoisomer composition of the chiral UV filter 4-methylbenzylidene camphor in environmental samples. Environ. Sci. Technol. 39, 3013–3019. Buser, H.-R., Poiger, T., and Muller, M. D. (1999). Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 33, 2529–2535. Cunningham, P., Afzal-Ahmed, I., and Naftalin, R. J. (2006). Docking studies show that D-glucose and quercetin slide through the transporter GLUT1. J. Biol. Chem. 281, 5797–5803. Cushny, A. R. (1903). Atropine and the hyoscyamines—A study of the action of optical isomers. J. Physiol. 30, 176–194. Damaj, M. I., Carroll, F. I., Eaton, J. B., Navarro, H. A., Blough, B. E., Mirza, S., Lukas, R. J., and Martin, B. R. (2004). Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol. Pharmacol. 66, 675–682. 24 SMITH De Flora, S., Cesarone, C. F., Balansky, R. M., Albini, A., D’Agostini, F., Bennicelli, C., Bagnasco, M., Camoirano, A., Scatolini, L., and Rovida, A. (1995). Chemopreventive properties and mechanisms of N-Acetylcysteine. The experimental background. J. Cell. Biochem. Suppl. 22, 33–41. Dehennin, L. (1994). On the origin of physiologically high ratios of urinary testosterone to epitestosterone: Consequences for reliable detection of testosterone administration by male athletes. J. Endocrinol. 142, 353–360. Delaney, C. E., Hopkins, S. P., and Addison, C. L. (2007). Supplementation with l-carnitine does not reduce the efficacy of epirubicin treatment in breast cancer cells. Cancer Lett. 252, 195–207. Delva, P., Degan, M., Pastori, C., Faccini, G., and Lechi, A. (2002). Glucoseinduced alterations of intracellular ionized magnesium in human lymphocytes. Life Sci. 71, 2119–2135. Dencker, L., and Eriksson, P. (1998). Susceptibility in utero and upon neonatal exposure. Food Addit. Contam. 15(Suppl), 37–43. Dickerson, R. E., Drew, H. R., Conner, B. N., Wing, R. M., Fratini, A. V., and Kopka, M. L. (1982). The anatomy of A-, B-, and Z-DNA. Science 216, 475–485. Dorey, E. (2000). Chiral drugs viable, despite failure. Nat. Biotechnol. 18, 1239–1240. Eap, C. B., Crettol, S., Rougier, J. S., Schlapfer, J., Sintra Grilo, L., Deglon, J. J., Besson, J., Croquette-Krokar, M., Carrupt, P. A., and Abriel, H. (2007). Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin. Pharmacol. Ther. 81, 719–728. Eap, C. B., Lessard, E., Baumann, P., Brawand-Amey, M., Yessine, M. A., O’Hara, G., and Turgeon, J. (2003). Role of CYP2D6 in the stereoselective disposition of venlafaxine in humans. Pharmacogenetics 13, 39–47. Edvardsen, Ø., and Dahl, S. (1991). Molecular structure and dynamics of acetylcholine. J. Neural Transm. 83, 157–170. Ehret, G. B., Voide, C., Gex-Fabry, M., Chabert, J., Shah, D., Broers, B., Piguet, V., Musset, T., Gaspoz, J. M., Perrier, A., et al. (2006). Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch. Intern. Med. 166, 1280–1287. Ehrlich, P., and Einhorn, A. (1894). Ueber die physiologische Wirkung der Verbindungen der Cocaı¨nreihe. Berichte d. deutsch. chem. Gesellsch. 27, 1870–1873. Eikel, D., Hoffmann, K., Zoll, K., Lampen, A., and Nau, H. (2006). S-2-pentyl4-pentynoic hydroxamic acid and its metabolite s-2-pentyl-4-pentynoic acid in the NMRI-exencephaly-mouse model: Pharmacokinetic profiles, teratogenic effects, and histone deacetylase inhibition abilities of further valproic acid hydroxamates and amides. Drug Metab. Dispos. 34, 612–620. Engel, J., Kristen, G., Schaefer, A., and von Schlichtegroll, A. (1986). Mefenorex (Rondimen). Drug Alcohol Depend. 17, 229–234. Eriksson, T., Bjorkman, S., and Hoglund, P. (2001). Clinical pharmacology of thalidomide. Eur. J. Clin. Pharmacol. 57, 365–376. Eriksson, T., Bjorkman, S., Roth, B., and Hoglund, P. (2000). Intravenous formulations of the enantiomers of thalidomide: Pharmacokinetic and initial pharmacodynamic characterization in man. J. Pharm. Pharmacol. 52, 807–817. Eisai, Inc. (2007). PanretinÒ (alitretinoin) Gel 0.1% (For Topical Use Only) [Healthcare Professional Information]. Eisai, Inc, Woodcliff Lake, NJ. European Medicines Agency (EMEA). (2008). Pre-authorisation evaluation of medicines for human use. Withdrawal Assessment Report for Voraxaze. Report number: EMEA/CHMP/171907/2008. European Medicines Agency (EMEA). London, UK. Fabro, S., Smith, R. L., and Williams, R. T. (1967). Toxicity and teratogenicity of optical isomers of thalidomide. Nature 215, 296. Fine, K. D., Santa Ana, C. A., Porter, J. L., and Fordtran, J. S. (1993). Effect of D-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology 105, 1117–1125. Fischer, E. (1891). Uber die Configuration des Traubenzuckers. und seiner Isomeren [On the configuration of grape sugar and its isomers]. Berichte d. deutsch. chem. Gesellsch. 24, 1836–1845. Fitos, I., Visy, J., and Kardos, J. (2002). Stereoselective kinetics of warfarin binding to human serum albumin: Effect of an allosteric interaction. Chirality 14, 442–448. Fitos, I., Visy, J., Zsila, F., Mady, G., and Simonyi, M. (2007). Conformation selectivity in the binding of diazepam and analogues to alpha1-acid glycoprotein. Bioorg. Med. Chem. 15, 4857–4862. Flombaum, C. D., and Meyers, P. A. (1999). High-dose leucovorin as sole therapy for methotrexate toxicity. J. Clin. Oncol. 17, 1589–1594. Fono, L. J., and Sedlak, D. L. (2005). Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters. Environ. Sci. Technol. 39, 9244–9252. Forest Pharmaceuticals, Inc. (2009). LexaproÒ (escitalopram oxalate) Tablets. LexaproÒ (escitalopram oxalate) Oral Solution Prescribing Information. Forest Pharmaceuticals, Inc., Subsidiary of Forest Laboratories, Inc., St. Louis, MO. Available at: http://www.frx.com/pi/lexapro_pi.pdf. Accessed May 18, 2009. Fraser, T. R. (1863). On the characters, actions, and therapeutic uses of the bean of Calabar. Edin. Med. J. 9, 36– 56; 123–132; 235–248. Frey, W. (1918). Uber Vorhofflimmern beim Menschen und seine Beseitigung durch Chinidin. Berl. Klin. Wchschr. 50, 450–452. Gal, J. (2008). The discovery of biological enantioselectivity: Louis Pasteur and the fermentation of tartaric acid, 1857—A review and analysis 150 yr later. Chirality 20, 5–19. Garcia Quetglas, E., Azanza, J. R., Cardenas, E., Sadaba, B., and Campanero, M. A. (2007). Stereoselective pharmacokinetic analysis of tramadol and its main phase I metabolites in healthy subjects after intravenous and oral administration of racemic tramadol. Biopharm. Drug Dispos. 28, 19–33. Gerber, J. G., Rhodes, R. J., and Gal, J. (2004). Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality 16, 36–44. Germain, P., Chambon, P., Eichele, G., Evans, R. M., Lazar, M. A., Leid, M., De Lera, A. R., Lotan, R., Mangelsdorf, D. J., and Gronemeyer, H. (2006a). International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58, 712–725. Germain, P., Chambon, P., Eichele, G., Evans, R. M., Lazar, M. A., Leid, M., De Lera, A. R., Lotan, R., Mangelsdorf, D. J., and Gronemeyer, H. (2006b). International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 58, 760–772. Gidal, B. E., Privitera, M. D., Sheth, R. D., and Gilman, J. T. (1999). Vigabatrin: A novel therapy for seizure disorders. Ann. Pharmacother. 33, 1277–1286. Good, E. E., and Ware, G. W. (1969). Effects of insecticides on reproduction in the laboratory mouse: IV. Endrin and dieldrin. Toxicol. Appl. Pharmacol. 14, 201–203. Goorin, A., Strother, D., Poplack, D., Letvak, L. A., George, M., and Link, M. (1995). Safety and efficacy of l-leucovorin rescue following high-dose methotrexate for osteosarcoma. Med. Pediatr. Oncol. 24, 362–367. Grdina, D. J., Murley, J. S., and Roberts, J. C. (1998). Effects of thiols on topoisomerase-II alpha activity and cell cycle progression. Cell Prolif. 31, 217–229. Gross, C. J., and Henderson, L. M. (1984). Absorption of D- and L-carnitine by the intestine and kidney tubule in the rat. Biochim. Biophys. Acta 772, 209–219. Grove, W. E. (1910). On the toxicity of dextro-, laevo- and inactive camphor. J. Pharmacol. Exp. Ther. 1, 445–456. CHIRAL TOXICOLOGY Grover, S., Biswas, P., Bhateja, G., and Kulhara, P. (2007). Escitalopramassociated hyponatremia. Psychiatry Clin. Neurosci. 61, 132–133. Haglund, P. (1996). Enantioselective separation of polychlorinated biphenyl atropisomers using chiral high-performance liquid chromatography. J. Chromatogr. A 724, 219–228. Hanada, K., Ohta, T., Hirai, M., Arai, M., and Ogata, H. (2000). Enantioselective binding of propranolol, disopyramide, and verapamil to human alpha(1)-acid glycoprotein. J. Pharm. Sci. 89, 751–757. Hao, H., Wang, G., and Sun, J. (2005). Enantioselective pharmacokinetics of ibuprofen and involved mechanisms. Drug Metab. Rev. 37, 215–234. Harris, L. W., Anderson, D. R., Pastelak, A. M., and Vanderpool, B. (1990). Acetylcholinesterase inhibition by (þ)physostigmine and efficacy against lethality induced by soman. Drug Chem. Toxicol. 13, 241–248. Harrison, P. M., Wendon, J. A., Gimson, A. E., Alexander, G. J., and Williams, R. (1991). Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N. Engl. J. Med. 324, 1852–1857. Hasinoff, B. B. (1994). Stereoselective hydrolysis of ICRF-187 (dexrazoxane) and ICRF-186 by dihydropyrimidine amidohydrolase. Chirality 6, 213–215. Hasinoff, B. B., and Aoyama, R. G. (1999). Stereoselective metabolism of dexrazoxane (ICRF-187) and levrazoxane (ICRF-186). Chirality 11, 286–290. Hasinoff, B. B., Kuschak, T. I., Yalowich, J. C., and Creighton, A. M. (1995). A QSAR study comparing the cytotoxicity and DNA topoisomerase II inhibitory effects of bisdioxopiperazine analogs of ICRF-187 (dexrazoxane). Biochem. Pharmacol. 50, 953–958. Hathcock, J. N., and Shao, A. (2006). Risk assessment for carnitine. Regul. Toxicol. Pharmacol. 46, 23–28. Hauck, R. S., and Nau, H. (1992). The enantiomers of the valproic acid analogue 2-n-propyl-4-pentynoic acid (4-yn-VPA): Asymmetric synthesis and highly stereoselective teratogenicity in mice. Pharm. Res. 9, 850–855. Heger, W., Schmahl, H. J., Klug, S., Felies, A., Nau, H., Merker, H. J., and Neubert, D. (1994). Embryotoxic effects of thalidomide derivatives in the non-human primate callithrix jacchus. IV. Teratogenicity of micrograms/kg doses of the EM12 enantiomers. Teratog. Carcinog. Mutagen. 14, 115–122. Hempel, G., Lingg, R., and Boos, J. (2005). Interactions of carboxypeptidase G2 with 6S-leucovorin and 6R-leucovorin in vitro: Implications for the application in case of methotrexate intoxications. Cancer Chemother. Pharmacol. 55, 347–353. Heydorn, S., Menne, T., Andersen, K. E., Bruze, M., Svedman, C., Basketter, D., and Johansen, J. D. (2003). The fragrance hand immersion study—An experimental model simulating real-life exposure for allergic contact dermatitis on the hands. Contact Dermatitis 48, 324–330. Hill, R. K., and Newkome, G. R. (1969). The absolute configuration of physostigmine. Tetrahedron 25, 1249–1260. Hoekstra, P. F., Burnison, B. K., Neheli, T., and Muir, D. C. (2001). Enantiomer-specific activity of o,p’-DDT with the human estrogen receptor. Toxicol. Lett. 125, 75–81. Hoglund, P., Eriksson, T., and Bjorkman, S. (1998). A double-blind study of the sedative effects of the thalidomide enantiomers in humans. J. Pharmacokinet. Biopharm. 26, 363–383. Honein, M. A., Lindstrom, J. A., and Kweder, S. L. (2007). Can we ensure the safe use of known human teratogens?: The iPLEDGE test case. Drug Saf. 30, 5–15. Huska, M. T., Catalano, G., and Catalano, M. C. (2007). Serotonin syndrome associated with the use of escitalopram. CNS Spectr. 12, 270–274. Huth, P. J., Schmidt, M. J., Hall, P. V., Fariello, R. G., and Shug, A. L. (1981). The uptake of carnitine by slices of rat cerebral cortex. J. Neurochem. 36, 715–723. Hutt, A. J., and O’Grady, J. (1996). Drug chirality: A consideration of the significance of the stereochemistry of antimicrobial agents. J. Antimicrob. Chemother. 37, 7–32. 25 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (1999). d-Limonene (group 3). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 73, Some Chemicals that Cause Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances, pp. 307–327. World Health Organization International Agency for Research on Cancer, Lyons, France. Ihmsen, H., Geisslinger, G., and Schuttler, J. (2001). Stereoselective pharmacokinetics of ketamine: R(-)-ketamine inhibits the elimination of S(þ)-ketamine. Clin. Pharmacol. Ther. 70, 431–438. Ishida, K., Taira, S., Morishita, H., Kayano, Y., Taguchi, M., and Hashimoto, Y. (2008). Stereoselective oxidation and glucuronidation of carvedilol in human liver and intestinal microsomes. Biol. Pharm. Bull. 31, 1297–1300. IUPAC. (1997). Compendium of Chemical Terminology. 2nd edn(the ‘‘Gold Book’’). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford. XML on-line corrected version (2006). Created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. goldbook.iupac.org Jaffe, N., Jorgensen, K., Robertson, R., George, M., Letvak, L., and Barrett, G. (1993). Substitution of l-leucovorin for d,l-leucovorin in the rescue from high-dose methotrexate treatment in patients with osteosarcoma. Anticancer Drugs 4, 559–564. Jiang, B., Wang, H., Fu, Q. M., and Li, Z. Y. (2008). The chiral pyrethroid cycloprothrin: Stereoisomer synthesis and separation and stereoselective insecticidal activity. Chirality 20, 96–102. Jick, S. S., Terris, B. Z., and Jick, H. (1993). First trimester topical tretinoin and congenital disorders. Lancet 341, 1181–1182. Jirovsky, D., Lemr, K., Sevcik, J., Smysl, B., and Stransky, Z. (1998). Methamphetamine—Properties and analytical methods of enantiomer determination. Forensic Sci. Int. 96, 61–70. Jones, W. J., Mazur, C. S., Kenneke, J. F., and Garrison, A. W. (2007). Enantioselective microbial transformation of the phenylpyrazole insecticide fipronil in anoxic sediments. Environ. Sci. Technol. 41, 8301–8307. Kaminsky, L. S., and Zhang, Z. Y. (1997). Human P450 metabolism of warfarin. Pharmacol. Ther. 73, 67–74. Kania-Korwel, I., Vyas, S. M., Song, Y., and Lehmler, H. (2008). Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J. Chromatogr. A 1207, 146–154. Karasek, L., Hajslova, J., Rosmus, J., and Huhnerfuss, H. (2007). Methylsulfonyl PCB and DDE metabolites and their enantioselective gas chromatographic separation in human adipose tissues, seal blubber and pelican muscle. Chemosphere 67, S22–27. Kasparkova, J., Marini, V., Bursova, V., and Brabec, V. (2008). Biophysical studies on the stability of DNA intrastrand cross-links of transplatin. Biophys. J. 95, 4361–4371. Kauffman, R. E., Lieh-Lai, M. W., Uy, H. G., and Aravind, M. K. (1999). Enantiomer-selective pharmacokinetics and metabolism of ketorolac in children. Clin. Pharmacol. Ther. 65, 382–388. Kawabuchi, M., Boyne, A. F., Deshpande, S. S., Cintra, W. M., Brossi, A., and Albuquerque, E. X. (1988). Enantiomer (þ)physostigmine prevents organophosphate-induced subjunctional damage at the neuromuscular synapse by a mechanism not related to cholinesterase carbamylation. Synapse 2, 139–147. Kean, W. F., Lock, C. J., and Howard-Lock, H. E. (1991). Chirality in antirheumatic drugs. Lancet 338, 1565–1568. Keays, R., Harrison, P. M., Wendon, J. A., Forbes, A., Gove, C., Alexander, G. J., and Williams, R. (1991). Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: A prospective controlled trial. BMJ 303, 1026–1029. Kerper, L. E., Ballatori, N., and Clarkson, T. W. (1992). Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am. J. Physiol. 262, R761–R765. 26 SMITH Kharasch, E. D., Mitchell, D., and Coles, R. (2008). Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J. Clin. Pharmacol. 48, 464–474. Li, Z. Y., Zhang, Z. C., Zhang, L., and Leng, L. (2008). Stereo and enantioselective degradation of beta-Cypermethrin and beta-Cyfluthrin in soil. Bull. Environ. Contam. Toxicol. 80, 335–339. Kimura, T., Yamano, H., Tanaka, A., Matsumura, T., Ueda, M., Ogawara, K., and Higaki, K. (2002). Transport of D-glucose across cultured stratified cell layer of human oral mucosal cells. J. Pharm. Pharmacol. 54, 213–219. Liedtke, A. J., Nellis, S. H., Whitesell, L. F., and Mahar, C. Q. (1982). Metabolic and mechanical effects using L- and D-carnitine in working swine hearts. Am. J. Physiol. 243, H691–H697. Knihinicki, R. D., Day, R. O., and Williams, K. M. (1991). Chiral inversion of 2-arylpropionic acid non-steroidal anti-inflammatory drugs—II. Racemization and hydrolysis of (R)- and (S)-ibuprofen-CoA thioesters. Biochem. Pharmacol. 42, 1905–1911. Kolliker, S., and Oehme, M. (2004). Structure elucidation of nanogram quantities of unknown designer drugs based on phenylalkylamine derivates by ion trap multiple mass spectrometry. Anal. Bioanal. Chem. 378, 1294–1304. Liedtke, A., Nellis, S., and Whitesell, L. (1981). Effects of carnitine isomers on fatty acid metabolism in ischemic swine hearts. Circ. Res. 48, 859–866. Lin, M. C., Hwang, M. T., Chang, H. G., Lin, C. S., and Lin, G. (2007). Benzene1,2-, 1,3-, and 1,4-di-N-substituted carbamates as conformationally constrained inhibitors of acetylcholinesterase. J. Biochem. Mol. Toxicol. 21, 348–353. Konwick, B. J., Garrison, A. W., Black, M. C., Avants, J. K., and Fisk, A. T. (2006). Bioaccumulation, biotransformation, and metabolite formation of fipronil and chiral legacy pesticides in rainbow trout. Environ. Sci. Technol. 40, 2930–2936. Liu, W., Gan, J., Lee, S., and Werner, I. (2005a). Isomer selectivity in aquatic toxicity and biodegradation of bifenthrin and permethrin. Environ. Toxicol. Chem. 24, 1861–1866. Kraemer, T., Roditis, S. K., Peters, F. T., and Maurer, H. H. (2003). Amphetamine concentrations in human urine following single-dose administration of the calcium antagonist prenylamine-studies using fluorescence polarization immunoassay (FPIA) and GC-MS. J. Anal. Toxicol. 27, 68–73. Krantz, M. J., Martin, J., Stimmel, B., Mehta, D., and Haigney, M. C. (2009). QTc interval screening in methadone treatment. Ann. Intern. Med. 150, 387–95. Lammer, E. J., Chen, D. T., Hoar, R. M., Agnish, N. D., Benke, P. J., Braun, J. T., Curry, C. J., Fernhoff, P. M., Grix, A. W., Jr., and Lott, I. T. (1985). Retinoic acid embryopathy. N. Engl. J. Med. 313, 837–841. Lao, Y., Yu, N., Kassie, F., Villalta, P. W., and Hecht, S. S. (2007). Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N#-nitrosonornicotine. Chem. Res. Toxicol. 20, 246–256. Larsson, C., Ellerichmann, T., Huhnerfuss, H., and Bergman, A. (2002). Chiral PCB methyl sulfones in rat tissues after exposure to technical PCBs. Environ. Sci. Technol. 36, 2833–2838. Le Bel, J. A. (1874). Sur des relation qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire des leurs dissolutions [On the relations which exist between the atomic formulas of organic compounds and the rotatory power of their solutions]. Bull. Soc. Chim. 22, 337–347. Lee, W., Chan, M., Tam, W., and Hung, M. (2007). The application of capillary electrophoresis for enantiomeric separation of N,N-dimethylamphetamine and its related analogs: Intelligence study on N,N-dimethylamphetamine samples in crystalline and tablet forms. Forensic Sci. Int. 165, 71–77. Leung, G. P., Man, R. Y., and Tse, C. M. (2005). D-Glucose upregulates adenosine transport in cultured human aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 288, H2756–62. Lewis, D. L., Garrison, A. W., Wommack, K. E., Whittemore, A., Steudler, P., and Melillo, J. (1999). Influence of environmental changes on degradation of chiral pollutants in soils. Nature 401, 898–901. Lheureux, P. E., Penaloza, A., Zahir, S., and Gris, M. (2005). Science review: Carnitine in the treatment of valproic acid-induced toxicity—What is the evidence? Crit. Care 9, 431–440. Li, J., Zhang, G., Qi, S., Li, X., and Peng, X. (2006). Concentrations, enantiomeric compositions, and sources of HCH, DDT and chlordane in soils from the Pearl River Delta, South China. Sci. Total Environ. 372, 215–224. Li, P., and Akk, G. (2008). The insecticide fipronil and its metabolite fipronil sulphone inhibit the rat alpha1beta2gamma2L GABA(A) receptor. Br. J. Pharmacol. 155, 783–974. Liu, J., and Liu, R. H. (2002). Enantiomeric composition of abused amine drugs: Chromatographic methods of analysis and data interpretation. J. Biochem. Biophys. Methods 54, 115–146. Liu, W., Gan, J. J., and Qin, S. (2005b). Separation and aquatic toxicity of enantiomers of synthetic pyrethroid insecticides. Chirality 17(Suppl), S127–S133. Loureiro, K. D., Kao, K. K., Jones, K. L., Alvarado, S., Chavez, C., Dick, L., Felix, R., Johnson, D., and Chambers, C. D. (2005). Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am. J. Med. Genet. A 136, 117–121. Lu, L., Leonessa, F., Baynham, M. T., Clarke, R., Gimenez, F., Pham, Y. T., Roux, F., and Wainer, I. W. (2001). The enantioselective binding of mefloquine enantiomers to P-glycoprotein determined using an immobilized P-glycoprotein liquid chromatographic stationary phase. Pharm. Res. 18, 1327–1330. Lurie, I. S., and Cox, K. A. (2005). Rapid chiral separation of dextro and levomethorphan using capillary electrophoresis with dynamically coated capillaries. DEA Microgram J. 3, 138. Lurie, I. S. (1998). Capillary electrophoresis of illicit drug seizures. Forensic Sci. Int. 92, 125–136. MacLean, D. A., Ettinger, S. M., Sinoway, L. I., and Lanoue, K. F. (2001). Determination of muscle-specific glucose flux using radioactive stereoisomers and microdialysis. Am. J. Physiol. Endocrinol. Metab. 280, E187–E192. Majerus, T. C., Dasta, J. F., Bauman, J. L., Danziger, L. H., and Ruffolo, R. R., Jr. (1989). Dobutamine: Ten years later. Pharmacotherapy 9, 245–259. Marchan, V., Pedroso, E., and Grandas, A. (2004). Insights into the reaction of transplatin with DNA and proteins: Methionine-mediated formation of histidine-guanine trans-Pt(NH3)2 cross-links. Chemistry 10, 5369–5375. Massicotte, A. (2008). Contrast medium-induced nephropathy: Strategies for prevention. Pharmacotherapy. 28, 1140–1150. Matherly, L. H., and Hou, Z. (2008). Chapter 5 structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 79C, 145–184. Matsuoka, M., and Igisu, H. (1993). Comparison of the effects of L-carnitine, D-carnitine and acetyl-L-carnitine on the neurotoxicity of ammonia. Biochem. Pharmacol. 46, 159–164. McCaffery, P. J., Adams, J., Maden, M., and Rosa-Molinar, E. (2003). Too much of a good thing: Retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur. J. Neurosci. 18, 457–472. Mehvar, R., Brocks, D. R., and Vakily, M. (2002). Impact of stereoselectivity on the pharmacokinetics and pharmacodynamics of antiarrhythmic drugs. Clin. Pharmacokinet. 41, 533–558. Mendelson, J., Uemura, N., Harris, D., Nath, R. P., Fernandez, E., Jacob, P., 3rd., Everhart, E. T., and Jones, R. T. (2006). Human pharmacology of the methamphetamine stereoisomers. Clin. Pharmacol. Ther. 80, 403–420. CHIRAL TOXICOLOGY Mile, B. (2005). Chemistry in Court. Chromatographia 62, 3–9. Militante, J., Ma, B. W., Akk, G., and Steinbach, J. H. (2008). Activation and block of the adult muscle-type nicotinic receptor by physostigmine: Singlechannel studies. Mol. Pharmacol. 74, 764–776. Mills, S. A., 3rd., Thal, D. I., and Barney, J. (2007). A summary of the 209 PCB congener nomenclature. Chemosphere 68, 1603–1612. Miura, M. (2006). Enantioselective disposition of lansoprazole and rabeprazole in human plasma. Yakugaku Zasshi. 126, 395–402. Mocquet, V., Kropachev, K., Kolbanovskiy, M., Kolbanovskiy, A., Tapias, A., Cai, Y., Broyde, S., Geacintov, N. E., and Egly, J. M. (2007). The human DNA repair factor XPC-HR23B distinguishes stereoisomeric benzo[a]pyrenyl-DNA lesions. EMBO J. 26, 2923–2932. Mokrzan, E. M., Kerper, L. E., Ballatori, N., and Clarkson, T. W. (1995). Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J. Pharmacol. Exp. Ther. 272, 1277–1284. Morley, N., Curnow, A., Salter, L., Campbell, S., and Gould, D. (2003). Nacetyl-L-cysteine prevents DNA damage induced by UVA, UVB and visible radiation in human fibroblasts. J. Photochem. Photobiol. B. 72, 55–60. Mouridsen, H. T., Langer, S. W., Buter, J., Eidtmann, H., Rosti, G., de Wit, M., Knoblauch, P., Rasmussen, A., Dahlstrom, K., Jensen, P. B., et al. (2007). Treatment of anthracycline extravasation with Savene (dexrazoxane): Results from two prospective clinical multicentre studies. Ann. Oncol. 18, 546–550. Mroszczak, E., Combs, D., Chaplin, M., Tsina, I., Tarnowski, T., Rocha, C., Tam, Y., Boyd, A., Young, J., and Depass, L. (1996). Chiral kinetics and dynamics of ketorolac. J. Clin. Pharmacol. 36, 521–539. Musshoff, F. (2000). Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine. Drug Metab. Rev. 32, 15–44. Musshoff, F., and Kraemer, T. (1998). Identification of famprofazone ingestion. Int. J. Legal Med. 111, 305–308. Nahshoni, E., Weizman, A., Shefet, D., and Pik, N. (2004). A case of hyponatremia associated with escitalopram. J. Clin. Psychiatry 65, 1722. Narawa, T., Tsuda, Y., and Itoh, T. (2007). Chiral recognition of amethopterin enantiomers by the reduced folate carrier in Caco-2 cells. Drug Metab. Pharmacokinet. 22, 33–40. Nau, H., Hauck, R. S., and Ehlers, K. (1991). Valproic acid-induced neural tube defects in mouse and human: Aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol. Toxicol. 69, 310–321. Neupert, W., Brugger, R., Euchenhofer, C., Brune, K., and Geisslinger, G. (1997). Effects of ibuprofen enantiomers and its coenzyme A thioesters on human prostaglandin endoperoxide synthases. Br. J. Pharmacol. 122, 487–492. Newman, C. M., Warren, J. B., Taylor, G. W., Boobis, A. R., and Davies, D. S. (1990). Rapid tolerance to the hypotensive effects of glyceryl trinitrate in the rat: Prevention by N-acetyl-L- but not N-acetyl-D-cysteine. Br. J. Pharmacol. 99, 825–829. Nezel, T., Mu¨ller-Plathe, F., Mu¨ller, M. D., and Buser, H. (1997). Theoretical considerations about chiral PCBs and their methylthio and methylsulfonyl metabolites being possibly present as stable enantiomers. Chemosphere 35, 1895–1906. Nickalls, R. W., and Nickalls, E. A. (1988). The first use of physostigmine in the treatment of atropine poisoning. A translation of Kleinwachter’s paper entitled ‘Observations on the effect of Calabar bean extract as an antidote to atropine poisoning’. Anaesthesia 43, 776–779. Nicklasson, M., Bjorkman, S., Roth, B., Jonsson, M., and Hoglund, P. (2002). Stereoselective metabolism of pentoxifylline in vitro and in vivo in humans. Chirality 14, 643–652. Nikolai, L. N., McClure, E. L., MacLeod, S. L., and Wong, C. S. (2006). Stereoisomer quantification of the -blocker drugs atenolol, metoprolol, and 27 propranolol in wastewaters by chiral high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1131, 103–109. Nillos, M. G., Rodriguez-Fuentes, G., Gan, J., and Schlenk, D. (2007). Enantioselective acetylcholinesterase inhibition of the organophosphorous insecticides profenofos, fonofos, and crotoxyphos. Environ. Toxicol. Chem. 26, 1949–1954. Ofori-Adjei, D., Ericsson, O., Lindstrom, B., and Sjoqvist, F. (1986). Protein binding of chloroquine enantiomers and desethylchloroquine. Br. J. Clin. Pharmacol. 22, 356–358. Olsen, D. G., Dart, R. C., and Robinett, M. (2004). Severe serotonin syndrome from escitalopram overdose. Clin. Toxicol. 42, 744– 745; (Abstract). Osman, A., Enstrom, C., and Lindahl, T. L. (2007). Plasma S/R ratio of warfarin co-varies with VKORC1 haplotype. Blood Coagul. Fibrinolysis 18, 293–296. Overmyer, J. P., Rouse, D. R., Avants, J. K., Garrison, A. W., Delorenzo, M. E., Chung, K. W., Key, P. B., Wilson, W. A., and Black, M. C. (2007). Toxicity of fipronil and its enantiomers to marine and freshwater non-targets. J. Environ. Sci. Health B. 42, 471–480. Pagano, D. A., Yagen, B., Hernandez, O., Bend, J. R., and Zeiger, E. (1982). Mutagenicity of (R) and (S) styrene 7,8-oxide and the intermediary mercapturic acid metabolites formed from styrene 7,8-oxide. Environ. Mutagen. 4, 575–584. Page, R. L., 2nd., Ruscin, J. M., Bainbridge, J. L., and Brieke, A. A. (2008). Restless legs syndrome induced by escitalopram: Case report and review of the literature. Pharmacotherapy 28, 271–280. Paizs, B., and Simonyi, M. (1999). Ring inversion barrier of diazepam and derivatives: An ab initio study. Chirality 11, 651–658. Pakdeesusuk, U., Jones, W. J., Lee, C. M., Garrison, A. W., O’Niell, W. L., Freedman, D. L., Coates, J. T., and Wong, C. S. (2003). Changes in enantiomeric fractions during microbial reductive dechlorination of PCB132, PCB149, and araclor 1254 in Lake Hartwell sediment microcosms. Environ. Sci. Technol. 37, 1100–1107. Park, B. K. (1988). Warfarin: Metabolism and mode of action. Biochem. Pharmacol. 37, 19–27. Pasteur, L. (1857). Me´moire sur la fermentation alcoolique [Memoir on alcoholic fermentation]. C. R. Se´ances Acad. Sci. 45, 1032–1036. Pasteur, L. (1848). Me´moire sur la relation qui peut exister entre la forme crystalline et la composition chimique, et sur la cause de la polarisation rotatoire [Note on the relationship of crystalline form to chemical composition, and on the cause of rotatory polarization]. C. R. Se´ances Acad. Sci. 26, 535–538. Pearson, E. C., and Woosley, R. L. (2005). QT prolongation and torsades de pointes among methadone users: Reports to the FDA spontaneous reporting system. Pharmacoepidemiol. Drug Saf. 14, 747–753. Peck, G. L., Olsen, T. G., Yoder, F. W., Strauss, J. S., Downing, D. T., Pandya, M., Butkus, D., and Arnaud-Battandier, J. (1979). Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N. Engl. J. Med. 300, 329–333. Pereira, E. F., Hilmas, C., Santos, M. D., Alkondon, M., Maelicke, A., and Albuquerque, E. X. (2002). Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 53, 479–500. Pessah, I. N., Lehmler, H. J., Robertson, L. W., Perez, C. F., Cabrales, E., Bose, D. D., and Feng, W. (2009). Enantiomeric specificity of (-)2,2#,3,3#,6,6#-Hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem. Res. Toxicol. 22, 201–207. Petcher, T. J., and Pauling, P. (1973). Cholinesterase inhibitors: Structure of eserine. Nature 241, 277. Pham, Y. T., Regina, A., Farinotti, R., Couraud, P., Wainer, I. W., Roux, F., and Gimenez, F. (2000). Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochim. Biophys. Acta. 1524, 212–219. 28 SMITH Pictet, A., and Rotschy, A. (1904). Synthese des Nicotins. Berichte d. deutsch. chem. Gesellsch. 37, 1225–1235. Piper, T., Mareck, U., Geyer, H., Flenker, U., Thevis, M., Platen, P., and Schanzer, W. (2008). Determination of 13C/12C ratios of endogenous urinary steroids: Method validation, reference population and application to doping control purposes. Rapid Commun. Mass Spectrom. 22, 2161–2175. Pistolozzi, M., and Bertucci, C. (2008). Species-dependent stereoselective drug binding to albumin: A circular dichroism study. Chirality 20, 552–558. Popp, B. D., Hutchinson, D. S., Evans, B. A., and Summers, R. J. (2004). Stereoselectivity for interactions of agonists and antagonists at mouse, rat and human beta3-adrenoceptors. Eur. J. Pharmacol. 484, 323–331. Poulson, E. (1890). Beitra¨ge zur Kenntniss der pharmakologischen Gruppe des Cocains. Arch. Exp. Pathol. Pharmak. 27, 301–313. Prelog, V., and Helmchen, G. (1982). Basic principles of the CIP-system and proposals for a revision. Angewandte Chemie International Edition in English 21, 567–583. Pujals, S., Fernandez-Carneado, J., Ludevid, M. D., and Giralt, E. (2008). DSAP: A new, noncytotoxic, and fully protease resistant cell-penetrating peptide. ChemMedChem. 3, 296–301. Qin, S., Budd, R., Bondarenko, S., Liu, W., and Gan, J. (2006). Enantioselective degradation and chiral stability of pyrethroids in soil and sediment. J. Agric. Food Chem. 54, 5040–5045. Ranade, V. V., and Somberg, J. C. (2005). Chiral cardiovascular drugs: An overview. Am. J. Ther. 12, 439–459. Raymer, G. S., Hartman, D. E., Rowe, W. A., Werkman, R. F., and Koch, K. L. (2003). An open-label trial of L-glucose as a colon-cleansing agent before colonoscopy. Gastrointest. Endosc. 58, 30–35. Rebouche, C. J. (1983). Effect of dietary carnitine isomers and gammabutyrobetaine on L-carnitine biosynthesis and metabolism in the rat. J. Nutr. 113, 1906–1913. Reeves, D. (2007). Management of anthracycline extravasation injuries. Ann. Pharmacother. 41, 1238–1242. Reis, M., Cherma, M. D., Carlsson, B., and Bengtsson, F., and Task Force for TDM of Escitalopram in Sweden (2007). Therapeutic drug monitoring of escitalopram in an outpatient setting. Ther. Drug Monit. 29, 758–766. Reist, M., Carrupt, P. A., Francotte, E., and Testa, B. (1998). Chiral inversion and hydrolysis of thalidomide: Mechanisms and catalysis by bases and serum albumin, and chiral stability of teratogenic metabolites. Chem. Res. Toxicol. 11, 1521–1528. Rettie, A. E., and Tai, G. (2006). The pharmocogenomics of warfarin: Closing in on personalized medicine. Mol. Interv. 6, 223–227. Rietveld, E. C., van Gastel, F. J., Seutter-Berlage, F., and Zwanenburg, B. (1988). Glutathione conjugation and bacterial mutagenicity of racemic and enantiomerically pure cis- and trans-methyl epoxycinnamates. Arch. Toxicol. 61, 366–372. Rodriguez, A. T., Valtier, S., and Cody, J. T. (2004). Metabolic profile of famprofazone following multidose administration. J. Anal. Toxicol. 28, 432–438. Rossini, P. M., Marchionno, L., Gambi, D., Pirchio, M., Del Rosso, G., and Albertazzi, A. (1981). EMG changes in chronically dialyzed uraemic subjects undergoing d, 1-carnitine treatment. Ital. J. Neurol. Sci. 2, 255–262. Sarnstrand, B., Tunek, A., Sjodin, K., and Hallberg, A. (1995). Effects of Nacetylcysteine stereoisomers on oxygen-induced lung injury in rats. Chem. Biol. Interact. 94, 157–164. Scheepers, A., Joost, H. G., and Schurmann, A. (2004). The glucose transporter families SGLT and GLUT: Molecular basis of normal and aberrant function. JPEN J. Parenter. Enteral Nutr. 28, 364–371. Schilsky, R. L., and Ratain, M. J. (1990). Clinical pharmacokinetics of highdose leucovorin calcium after intravenous and oral administration. J. Natl. Cancer Inst. 82, 1411–1415. Schmahl, H. J., Nau, H., and Neubert, D. (1988). The enantiomers of the teratogenic thalidomide analogue EM 12: 1. Chiral inversion and plasma pharmacokinetics in the marmoset monkey. Arch. Toxicol. 62, 200–204. Schulze, J. J., Lundmark, J., Garle, M., Skilving, I., Ekstrom, L., and Rane, A. (2008). Doping test results dependent on genotype of uridine diphosphoglucuronosyl transferase 2B17, the major enzyme for testosterone glucuronidation. J. Clin. Endocrinol. Metab. 93, 2500–2506. Schwartz, J. B., Capili, H., and Wainer, I. W. (1994). Verapamil stereoisomers during racemic verapamil administration: Effects of aging and comparisons to administration of individual stereoisomers. Clin. Pharmacol. Ther. 56, 368–376. Scott, A. K. (1993). Stereoisomers and drug toxicity. The value of single stereoisomer therapy. Drug Saf. 8, 149–159. Shallenberger, R. S. (1997). Taste recognition chemistry. Pure Appl. Chem. 69, 659–666. Shapiro, L., Pastuszak, A., Curto, G., and Koren, G. (1997). Safety of firsttrimester exposure to topical tretinoin: Prospective cohort study. Lancet 350, 1143–1144. Shen, S., He, Y., and Zeng, S. (2007). Stereoselective regulation of MDR1 expression in Caco-2 cells by cetirizine enantiomers. Chirality 19, 485–490. Sherwood, R. F., Melton, R. G., Alwan, S. M., and Hughes, P. (1985). Purification and properties of carboxypeptidase G2 from Pseudomonas sp. strain RS-16. Use of a novel triazine dye affinity method. Eur. J. Biochem. 148, 447–453. Sirotnak, F. M., Chello, P. L., Moccio, D. M., Kisliuk, R. L., Combepine, G., Gaumont, Y., and Montgomery, J. A. (1979). Stereospecificity at carbon 6 of fomyltetrahydrofolate as a competitive inhibitor of transport and cytotoxicity of methotrexate in vitro. Biochem. Pharmacol. 28, 2993–2997. Smilkstein, M. J., Knapp, G. L., Kulig, K. W., and Rumack, B. H. (1988). Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 319, 1557–1562. Smith, S. W., and Nelson, L. S. (2008). Case files of the New York City Poison Control Center: Antidotal strategies for the management of methotrexate toxicity. J. Med. Toxicol. 4, 132–140. Spectrum Pharmaceuticals, Inc. (2008). Fusilev (Levoleucovorin) Injection, Powder, Lyophilized, for Solution for Intravenous Use, Prescribing Information. Spectrum Pharmaceuticals, Inc., Irvine CA. Srinivas, N. R. (2004). Role of stereoselective assays in bioequivalence studies of racemic drugs: Have we reached a consensus? J. Clin. Pharmacol. 44, 115–119. Stanley, J. K., Ramirez, A. J., Chambliss, C. K., and Brooks, B. W. (2007). Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 69, 9–16. Russell, S. (2007). Carnitine as an antidote for acute valproate toxicity in children. Curr. Opin. Pediatr. 19, 206–210. Stanley, J. K., Ramirez, A. J., Mottaleb, M., Chambliss, C. K., and Brooks, B. W. (2006). Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 25, 1780–1786. Sternlieb, I. (1966). Penicillamine and the nephrotic syndrome. JAMA 198, 1311–1312. Sacher, F., Ehmann, M., Gabriel, S., Graf, C., and Brauch, H. J. (2008). Pharmaceutical residues in the river Rhine—Results of a one-decade monitoring programme. J. Environ. Monit. 10, 664–670. Stewart, R. K., Serabjit-Singh, C. J., and Massey, T. E. (1996). Glutathione Stransferase-catalyzed conjugation of bioactivated aflatoxin B1 in rabbit lung and liver. Toxicol. Appl. Pharmacol. 140, 499–507. Ruffolo, R. R., Jr. (1987). The pharmacology of dobutamine. Am. J. Med. Sci. 294, 244–248. CHIRAL TOXICOLOGY 29 Stodgell, C. J., Ingram, J. L., O’Bara, M., Tisdale, B. K., Nau, H., and Rodier, P. M. (2006). Induction of the homeotic gene Hoxa1 through valproic acid’s teratogenic mechanism of action. Neurotoxicol. Teratol. 28, 617–624. U.S. Food and Drug Administration. (1987). Import Alert IA6635. Report number: IA#66–35. U.S. Food and Drug Administration, published online at http://www.fda.gov/ora/fiars/ora_import_ia6635.html (accessed April 1, 2009). Strolin Benedetti, M., Whomsley, R., Mathy, F. X., Jacques, P., Espie, P., and Canning, M. (2008). Stereoselective renal tubular secretion of levocetirizine and dextrocetirizine, the two enantiomers of the H1-antihistamine cetirizine. Fundam. Clin. Pharmacol. 22, 19–23. United Nations Office on Drugs and Crime. (2005). Methods for impurity profiling of heroin and cocaine. Report number: ST/NAR/35. United Nations, New York. Tagliaro, F., and Bortolotti, F. (2008). Recent advances in the applications of CE to forensic sciences (2005-2007). Electrophoresis 29, 260–268. Vakily, M., Mehvar, R., and Brocks, D. (2002). Stereoselective pharmacokinetics and pharmacodynamics of anti-asthma agents. Ann. Pharmacother. 36, 693–701. Tagliaro, F., Bortolotti, F., and Pascali, J. P. (2007). Current role of capillary electrophoretic/electrokinetic techniques in forensic toxicology. Anal. Bioanal. Chem. 388, 1359–1364. van Dalen, E. C., Caron, H. N., Dickinson, H. O., and Kremer, L. C. (2008). Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst. Rev. 2, CD003917. Tan, H., Cao, Y., Tang, T., Qian, K., Chen, W. L., and Li, J. (2008). Biodegradation and chiral stability of fipronil in aerobic and flooded paddy soils. Sci. Total Environ 407, 428–437. van’t Hoff, J. H. (1874). Voorstel tot Uitbreiding der Tegenwoordige in de Scheikunde gebruikte Structuurformules in de Ruimte, benevens een daarmee samenhangende Opmerking omtrent het Verband tusschen Optisch Actief Vermogen en chemische Constitutie van Organische Verbindingen [Proposal for the extension of current chemical structural formulas into space, together with related observation on the connection between optically active power and the chemical constitution of organic compounds]. Arch. Neerl. Sci. Exacts Nat. 9, 445–454. Tanabe, S. (1988). PCB problems in the future: Foresight from current knowledge. Environ. Pollut. 50, 5–28. Tanaka, M., Nakamura, F., Mizokawa, S., Matsumura, A., Matsumura, K., and Watanabe, Y. (2003). Role of acetyl-L-carnitine in the brain: Revealed by bioradiography. Biochem. Biophys. Res. Commun. 306, 1064–1069. Tatsumi, A., Kadobayashi, M., and Iwakawa, S. (2007). Effect of ethanol on the binding of warfarin enantiomers to human serum albumin. Biol. Pharm. Bull. 30, 826–829. Teo, S. K., Chen, Y., Muller, G. W., Chen, R. S., Thomas, S. D., Stirling, D. I., and Chandula, R. S. (2003). Chiral inversion of the second generation IMiD CC-4047 (ACTIMID) in human plasma and phosphate-buffered saline. Chirality 15, 348–351. Thielitz, A., and Gollnick, H. (2008). Topical retinoids in acne vulgaris: Update on efficacy and safety. Am. J. Clin. Dermatol. 9, 369–381. Tocco, D. J., deLuna, F. A., Duncan, A. E., Hsieh, J. H., and Lin, J. H. (1990). Interspecies differences in stereoselective protein binding and clearance of MK-571. Drug Metab. Dispos. 18, 388–392. Totah, R. A., Allen, K. E., Sheffels, P., Whittington, D., and Kharasch, E. D. (2007). Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J. Pharmacol. Exp. Ther. 321, 389–399. Tsoko, M., Beauseigneur, F., Gresti, J., Niot, I., Demarquoy, J., Boichot, J., Bezard, J., Rochette, L., and Clouet, P. (1995). Enhancement of activities relative to fatty acid oxidation in the liver of rats depleted of L-carnitine by D-carnitine and a gamma-butyrobetaine hydroxylase inhibitor. Biochem. Pharmacol. 49, 1403–1410. Tu, J., Blackwell, R. Q., and Lee, P. F. (1963). DL-penicillamine as a cause of optic axial neuritis. JAMA. 185, 83–86. U.S. Food and Drug Administration. (2008). Electronic Orange Book (Current through October 2008). Levobupivicaine. Application number 020997. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. U.S. Food and Drug Administration. (2006). News Release: FDA Warns Consumers about Brazilian Diet Pills Found to Contain Active Drug Ingredients. Emagrece Sim and Herbathin Dietary Supplements May be Harmful. U.S. Food and Drug Administration, published online January 13, 2006, http://www.fda.gov/bbs/topics/news/2006/NEW01298.html (accessed April 1, 2009). U.S. Food and Drug Administration. (2005). Celgene Corporation Briefing Material. RevlimidÒ (lenalidomide) briefing material. Oncologic Drugs Advisory Committee Meeting, September 14, 2005, AM Session. U.S. Food and Drug Administration, published online at http://www.fda.gov/ohrms/ dockets/ac/05/briefing/2005-4174B2_01_01-Celgene-Revlimid.pdf (accessed April 1, 2009). U.S. Food and Drug Administration. (1992). FDA’s Policy Statement on the Development of New Stereoisomeric Drugs. Fed. Regist. 57, 22249. Vari, G., and Beckson, M. (2007). Escitalopram-associated serotonin toxicity. J. Clin. Psychopharmacol. 27, 229–230. Vistoli, G., Pedretti, A., Testa, B., and Matucci, R. (2007). The conformational and property space of acetylcholine bound to muscarinic receptors: An entropy component accounts for the subtype selectivity of acetylcholine. Arch. Biochem. Biophys. 464, 112–121. Wainer, I. W., and Granvil, C. P. (1993). Stereoselective separations of chiral anticancer drugs and their application to pharmacodynamic and pharmacokinetic studies. Ther. Drug Monit. 15, 570–575. Waldo, A. L., Camm, A. J., deRuyter, H., Friedman, P. L., MacNeil, D. J., Pauls, J. F., Pitt, B., Pratt, C. M., Schwartz, P. J., and Veltri, E. P. (1996). Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 348, 7–12. Walgren, J. L., Mitchell, M. D., and Thompson, D. C. (2005). Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 35, 325–361. Walle, U. K., Pesola, G. R., and Walle, T. (1993). Stereoselective sulphate conjugation of salbutamol in humans: Comparison of hepatic, intestinal and platelet activity. Br. J. Clin. Pharmacol. 35, 413–418. Walle, U. K., Walle, T., Bai, S. A., and Olanoff, L. S. (1983). Stereoselective binding of propranolol to human plasma, alpha 1-acid glycoprotein, and albumin. Clin. Pharmacol. Ther. 34, 718–723. Walshe, J. M. (1992). Chirality of penicillamine. Lancet 339, 254. Wang, L., Liu, W., Yang, C., Pan, Z., Gan, J., Xu, C., Zhao, M., and Schlenk, D. (2007). Enantioselectivity in estrogenic potential and uptake of bifenthrin. Environ. Sci. Technol. 41, 6124–6128. Wass, M., and Evered, D. F. (1970). Transport of penicillamine across mucosa of the rat small intestine in vitro. Biochem. Pharmacol. 19, 1287–1295. Wiberg, K., Letcher, R., Sandau, C., Duffe, J., Norstrom, R., Haglund, P., and Bidleman, T. (1998). Enantioselective gas chromatography/mass spectrometry of methylsulfonyl PCBs with application to arctic marine mammals. Anal. Chem. 70, 3845–3852. Widemann, B. C., and Adamson, P. C. (2006). Understanding and managing methotrexate nephrotoxicity. Oncologist 11, 694–703. Widemann, B. C., Balis, F. M., O’Brien, M., Cole, D., Murphy, R., Montello, M. J., Shriner, D., Adams, J., and Adamson, P. C. (1998). Rescue with carboxypeptidase-G2 (CPDG2) and leucovorin (LV) for patients with high-dose methotrexate (HDMTX) induced renal failure. Proc. Am. Soc. Clin. Oncol. 17, 222a. 30 SMITH Williams, K. M. (1990). Enantiomers in arthritic disorders. Pharmacol. Ther. 46, 273–295. Williams, M. L., and Wainer, I. W. (2002). Role of chiral chromatography in therapeutic drug monitoring and in clinical and forensic toxicology. Ther. Drug Monit. 24, 290–296. Wilson, J. E., and Du Vigneaud, V. (1948). L-Penicillamine as a metabolic antagonist. Science 107, 653. Wilson, W. A., Konwick, B. J., Garrison, A. W., Avants, J. K., and Black, M. C. (2008). Enantioselective chronic toxicity of fipronil to Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 54, 36–43. Winkler, M., Lawrence, J. R., and Neu, T. R. (2001). Selective degradation of ibuprofen and clofibric acid in two model river biofilm systems. Water Res. 35, 3197–3205. Wolosker, H., Dumin, E., Balan, L., and Foltyn, V. N. (2008). D-amino acids in the brain: D-Serine in neurotransmission and neurodegeneration. FEBS J. 275, 3514–3526. Wong, B. K., Chan, H. C., and Corcoran, G. B. (1986a). Selective effects of Nacetylcysteine stereoisomers on hepatic glutathione and plasma sulfate in mice. Toxicol. Appl. Pharmacol. 86, 421–429. Wong, B. K., Galinsky, R. E., and Corcoran, G. B. (1986b). Dissociation of increased sulfation from sulfate replenishment and hepatoprotection in acetaminophen-poisoned mice by N-acetylcysteine stereoisomers. J. Pharm. Sci. 75, 878–880. Wong, C. S. (2006). Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal. Bioanal. Chem. 386, 544–558. World Anti-Doping Agency. (2007). The World Anti-Doping Code. The 2008 Prohibited List. International Standard. World Anti-Doping Agency Xu, M. X., Yan, J. H., Lu, S. Y., Li, X. D., Chen, T., Ni, M. J., Dai, H. F., and Cen, K. F. (2008b). Source identification of PCDD/Fs in agricultural soils near to a Chinese MSWI plant through isomer-specific data analysis. Chemosphere 71, 1144–1155. Yamada, T., Okada, T., Sakaguchi, K., Ohfune, Y., Ueki, H., and Soloshonok, V. A. (2006). Efficient asymmetric synthesis of novel 4substituted and configurationally stable analogues of thalidomide. Organic Lett. 8, 5625–5628. Yeung, D. T., Smith, J. R., Sweeney, R. E., Lenz, D. E., and Cerasoli, D. M. (2008). A gas chromatographic-mass spectrometric approach to examining stereoselective interaction of human plasma proteins with soman. J. Anal. Toxicol. 32, 86–91. Yu, Q. S., Pei, X. F., Holloway, H. W., Greig, N. H., and Brossi, A. (1997). Total syntheses and anticholinesterase activities of (3aS)-N(8)-norphysostigmine, (3aS)-N(8)-norphenserine, their antipodal isomers, and other N(8)substituted analogues. J. Med. Chem. 40, 2895–2901. Yuksel, F. V., Tuzer, V., and Goka, E. (2005). Escitalopram intoxication. Eur. Psychiatry. 20, 82. Zeidan, Q., Strauss, M., Porras, N., and Anselmi, G. (2002). Differential longterm subcellular responses in heart and liver to adriamycin stress. Exogenous L-carnitine cardiac and hepatic protection. J. Submicrosc. Cytol. Pathol. 34, 315–321. Zhang, J., Herman, E. H., and Ferrans, V. J. (1994). Effects of ICRF186 [(L)1,2-bis(3,5-dioxopiperazinyl-1-yl)propane] on the toxicity of doxorubicin in spontaneously hypertensive rats. Toxicology 92, 179–192. Wsol, V., Skalova, L., and Szotakova, B. (2004). Chiral inversion of drugs: Coincidence or principle? Curr. Drug Metab. 5, 517–533. Zhang, S. Y., Ueyama, J., Ito, Y., Yanagiba, Y., Okamura, A., Kamijima, M., and Nakajima, T. (2008). Permethrin may induce adult male mouse reproductive toxicity due to cis isomer not trans isomer. Toxicology 248, 136–141. Xu, C., Wang, J., Liu, W., Daniel Sheng, G., Tu, Y., and Ma, Y. (2008a). Separation and aquatic toxicity of enantiomers of the pyrethroid insecticide lambda-cyhalothrin. Environ. Toxicol. Chem. 27, 174–181. Zhou, S., Lin, K., Yang, H., Li, L., Liu, W., and Li, J. (2007). Stereoisomeric separation and toxicity of a new organophosphorus insecticide chloramidophos. Chem. Res. Toxicol. 20, 400–405.