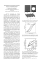mokht-2 _1
Transcription
mokht-2 _1
Effect of pH at Early Formed Structures in Cobalt Electrodeposition Mokhtari Salim, SAHARI ALI Salimmokhtari45@yahoo.mail University of Bouira 10000 Algeria Introduction In metals iron group, cobalt electrocrystallization has been far less studied compared to nickel, although electrodeposited cobalt and cobalt alloys are eligible materials for applications ranging from magnetic media and devices to wear and corrosion resistant coatings. [1]. The solution pH was shown to be the most important parameter in determining the structure of electrodeposited cobalt [2]. Pangarov and Rashkov [3] determined the relations between the main operative conditions and electrodeposits structure. They found that the cubic structure of cobalt (βCo) formation is favoured by low temperature, high current density and low pH. Polukarov [4] came to similar conclusions.. Most of the works found in the literature show that by increasing the pH solution the structure becomes completely the hexagonal closed packed structure (αCo)[5-6]. The purpose of the present work was to study, the influence of boric acid, and the effect of the pH solution on the electrochemical behaviour and on the electrodeposits morphology. In second hand the present paper gives the results of an investigation of the early stages of cobalt electrodeposition (the nucleation). I. Experimental details Electrochemical experiments were carried out in conventional three-electrode cell. The reference electrode was a saturated calomel electrode mounted in a Lugging capillary. All potentials are referred to this electrode. The counter electrode was platinum wire. A rectangular plate of platinum was used as working electrode. The exposited surface of this later electrode is about 0.4 cm2. Prior to each experiment, the working electrode was cleaned ultrasonically and sequentially in acetone, methanol and distilled water for 15 min, and finally activated in hydrochloric acid for 2 mn. Cobalt was deposited from two baths: The N°1 is used for studying the influence of H3BO3, the N°2 is for studying the effect of pH solution. The composition of each bath was shown in Table1.The electrodeposition of cobalt was performed at overpotential deposition (OPD) using a Volta Lab 40 potentiostat/galvanostat controlled by a PC. All baths 1 were stirred by nitrogen gas flow before electrodeposition of cobalt. Voltammetric experiments were carried out at 20 mV/s. X-ray ray diffraction (XRD) phase analysis was performed performed on a INEL CPS120 diffractometer. The CuKα radiation ( = 154066 pm) was used. The temperature was fixed at 20°C during all experiments. The surface morphology of the film was analyzed ex situ using a JEOL JSM6400 Scanning Electron Microscop equipped with an Oxford EDS system. TABLE I MOLAR COMPOSITION OF USED BATHS. Bath N°1 CoCl2 NaCl pH H3BO3 C/Mol/l 0.1 1.0 4.0 Varied Bath N°2 CoCl2 NaCl H3BO3 pH C/Mol/l 0.1 1.0 0.5 Varied 1 Surface morphology of electrodeposited Co films. The surface morphology of the Co deposited on Pt surface is shown in fig.5. The presence of Co was revealed by energy dispersive spectroscopy analysis (EDS). The SEM image corresponding to early stage of deposit at pH = 4. is schown in fig.5. The surface appears occupied by dispersed sub grains were seemed to grow uniformly with the same sizes, thus this distribution of grains proves the instantaneous nucleation nucleatio mechanism. a Fig.1: EDS analysis (a) for electrodeposits of Co at applied potential -1.0 1.0 V. 2 b Fig.2: SEM image (b) for electrodeposits of Co at applied potential -1.0 V. 2 Structural properties The effect of bath pH on the phase composition and microstructure of Co films was investigated by selecting the bath temperature at 20°C, and the applied potential was fixed at -1.0 V. Fig. 3 a, b and c shows the X-ray diffraction patterns of the films deposited at bath pH. 2, 3.1, and 4.0 respectively. At all selected pH range, several peaks were detected, indicating the polycrystalline structures. As can be seen at pH =2 the electrodeposited film consisted of mixture of Cohcp and Cofcc hcp (110) Pt (220) hcp (100) Pt (111) crystallographic phases, however their peaks were very small (Fig.3a). hcp (110) Pt (200) Intensity (arb. Units) hcp (100) c cfc(220) cfc (111) hcp (100) b a 40 50 60 70 80 2 θ (deg.) Fig.3: X-ray diffraction (XRD) patterns of electrodeposited Co from from solution shown in table 1.on Pt substrate. a: pH=2.0 b: pH = 3.1 c: pH = 4.0. If electrodeposition was conducted at pH = 3.1, and 4 the Cocfc variety was a tendency to disappear. The related Cohcp was considerably more pronounced, (Fig. 3 c, d), 3 Conclusion The influence of the boric acid and the effect of pH on Co electrodeposition have been evaluated in this present study. It was found that the boric acid alters strongly the polarization curves of Co electrodeposition. The major change in the voltammetry was a decrease in the hydrogen electrosorption activity. As a result, the potential region of proton discharge was extended to more negative potentials. At vertex potential region where proton discharge was under charge transfer control, the presence of boric acid leads to delay the current density of HER. This effect was attributed to adsorption of boric acid species. On the other hand, boric acid was found to affect the nucleation potential of Co. At low pH, Co structure is biphasic Cohcp + Cocfc with predominance of Cohcp depending on the rate of HER. The metastable Cocfc slowly disappeared with increasing the pH. The Co electrodeposition with operating potential of -1.0 V in pH bath = 4 was clearly under diffusion control. The nucleation mechanism was instantaneous diffusion controlled having a 3D-growth pattern. References [1] T. Osaka, Electrodeposition of highly functional thin films for magnetic recording devices of the next century Electrochim. Acta.45 (2000) 3311-3321, [2] H. Kersten, Influence of Hydrogene concentration on the Crystal Structure of Electrodeposited Cobalt. Physics. 2(1932).274-275. [3] N.A. Pangarov, St. Rashkov, Electrolytic deposition of alpha and beta cobalt,Compt. Rend. Acad. Bulgare Sci.13(1960) 439-442. [4] Yu.M. Polukarov, the structure and magnetic caracteristics of electrolytic deposits of ferromagnetic metals and alloys prepared under different conditions Russ. J. Phys. Chem.34 (1960) 68-71. [5] S. Armyanov, S.D. Vitkova,, Structure and magnetic properties of electrodeposited cobalt Surf. Technol. 7(1978) 319-329 . [6] G.I. Finch, H. Wilman, L. Yang, Disc. Faraday Soc. 1 (1947) 144-158 4