D-Mannitol Hexanitrate as Electron Acceptor in Charge Transfer
Transcription
D-Mannitol Hexanitrate as Electron Acceptor in Charge Transfer
BULLETIN D E L'ACADÉMIE
POLONAISE DES SCIENCES
S é r i e des sciences c h i m i q u e s
Volume
X V I I I , No. 7,
1970
ORGANIC
CHEMISTRY
D-Mannitol Hexanitrate as Electron Acceptor
in Charge Transfer Complexes
by
T. URBAŃSKI, В. H E T N A R S K I and W. POŁUDNIKIEWICZ
Presented ny T. URBAŃSKI
on March 2, 1970
As shown in our former papers [1, 2] nitric esters possess the property of electron
acceptors and hexanitrates of hexahydroxylic alcohols seem to be relatively strong
electron acceptors when tetramethyl-/>-phenylenediamine was used as an electron
donor. Among the nitrate in question D-mannitol hexanitrate seems to be parti
cularly interesting, in agreement with former findings of one of us [3].
The present paper is dedicated to a more detailed examination of charge transfer
(CT) complexes formed by D-mannitol hexanitrate ( M H N ) and tetramethyl-^-phenylenediamine (TMPD). As pointed out before [1,2], when dissolved in 1,2-dichloroethane, dichloromethane, or benzene, both components give high-intensity
violet colour produced by two absorption bands: at 570 and 620 nm, characteristic
of the T M P D cation [1,2,4].
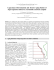
Both bands disappear with time and a new band at near 450 nm becomes evident
(Fig. 1). Measurements based on the method of continuous changes [5] indicate
that in the violet solutions two moles of T M P D originally reacted with one mole
of M H N . Next, after the 450 nm band was established, the donor/acceptor complex
was formed with the molar ratio 1:1. Fig. 2 gives Job curve taken 24 hours after
the preparation of the solution, i.e. when the original bands at 470 and 620 nm
practically disappeared.
It should be pointed out that the change of the colour and, therefore, the for
mation of a new yellow (absorption at 435—450 nm) complex occurred only in
solvents with a relatively low dielectric constant. When M H N and T M P D were
dissolved in methanol the original violet colour proved to be relatively stable and
the solution decolorised only after several days; no yellow complex was produced
there.
To examine the nature of the molecular additive compounds, experiments were
carried out in two-phase liquid system composed of one polar and another non-polar solvent, e.g. acetonitriles and н-heptane. After и-heptane was added to the
violet solution of M H N and T M P D in acetonitrile, л-heptane layer almost imme
diately turned yellow (absorption maximum at 435—450 nm). The intensity of the
band increased with time.
[405]
406
T. U r b a ń s k i et al.
The same was observed when methanol and 1,2-dichloroethane were used as
a polar and a low-polarity solvent, respectively.
We postulated that the yellow compound is a charge transfer complex and cal
culated equilibrium constants by means of the graphic method based on a modified
equation of Benesi—-Hildebrand applied for equimolar concentrations of com
ponents [9] (Fig. 3).
С
1
1
(1)
IID
C
K-E
Di
1.0,
as
0.6
0.4
700 A f n m )
Fig. 1. Change with time of electronic spectra
of M H N and T M P D in 1,2-dichloroethane
at 23°C. Concentration of each of the com
ponents, с = 0.002 mol/I ; 1 — immediately
after mixing, 2 — after 1 hour, 3 — after
24 hours
Fig. 2. Job curve of the M H N — T M P D complex
in 1,2 dichlorocthane after 24hrs.; D — Diffe
rence between the absorption of solution of the
complex and absorption of the components
measured separately, С — concentration of T M P D
in molar fractions of the sum of M H N plus
TMPD
where :
С — concentration of components, in mol/1.
K— equilibrium constant of the formation of the complex, in 1/mol.
£ — molar extinction coefficient, in l/cm mol,
D — absorbance of the solution at /.=450 nm.
The values of c in dichloromethane and 1,2-dichloroethane at different tem
peratures are collected in Table I.
C
K
TABLE
Solvent
Dielectric
CH C1
C1CH CH C1
2
2
2
2
9.08
10.36
К з°
лэр«
13.4
33.3
12.0
27.6
2
constant
17.3
59.3
The figures in the Table indicate that the formation constant of the complex
is lowered with temperature — the feature typical of the CT complexes.
u-Mannitol
Hexanitrate
as Electron
Acceptor
in CT
Complexes
407
C
The increase of the dielectric constants increases the values of K. This indicates
a close connection between the concentration of the T M P D cation (formed imme
diately after the solutions of T M P D and M H N were brought together) and the
concentration of the complex absorbing at 435—450 nm. When a solvent with
high dielectric constant is added the complex dissociates into ions. This was proved
by the decrease of the electric resistance in /г-heptane and in mixtures of /г-heptane
with 1,2-dichloroethane and methanol (Fig. 4).
Conclusions
We are suggesting the diagram for our reactions as follows:
CH
СНз
3
CH ^
^СНз
3
N
N
CH,ON0
I
C H , O N O , " 20
2
I
fast
(CHON0 )
I
CH ON0
2
2
4
I
(CHONO,)
I
CH,ON0
+ 2
2
I
N
/ "
\
СНз
СНз
2
+ 2
4
CHc
N
* \
/
N
CH _
4
(A)
"СНз^ ^ННз"
СНз^
Ń
I
СНз
^СНз
Ń
I
+
с н У
\ : h
СНз
3
N
/ " \
/
N
II
/ ч
slow
N
2©
СНз
\
N
сн
_СН ^
3
3
\ т Н
3
.
(В)
"СНз^
сн
29
3
2
2
CH ON0
2
2
4
3
N
N
C H O N 0 • 20
I
(CHONO,)
I
CH ON0
C H " 2©
СНз^
+
2
(ŒON0 )
I
CH ON0
2
2
" 29
4
2
2
N
N
СНз^
(С)
\ : Н
3
СНз
.
СНз.
(D)
When M H N and T M P D are brought together in a solvent a fast reaction leading
to the violet salt (A) occurs (see the diagram). It was formed by two T M P D cations
and one M H N anion. Tn a low-polarity solvents (dichloromethane, 1,2-dichloro-
T. U r b a ń s k i et al.
408
3
Q*10 {Q)
Fig. 3. Graphie presentation of figures from E q . (I)
for the complex M H N / T M P D (л = 450 nm) in 1,2
dichloroethane; d — absorption of the reaction mixture
Fig. 4. Change of resistance of the
solution of M H N in n heptane/1,2
dichloroethane against concentra
tion of methanol added
ethane) the system undergoes disproportionation (B) (a slow reaction confirmed
by TLC) and a new yellow complex (C, D) is formed. Here the ratio of compo
nents used was 1:1.
It was found that the formation of the complex (C, D) can be accelerated by
the addition of an excess M H N .
It should be pointed out that in the earlier work of one of us [3] the molar ratio
of the acceptor (MHN) to the aromatic donor was found to be 1:2 as in salts (A).
The high ionisation potential of the formerly used donors and probably the experi
mental conditions (no solvent and a relatively high temperature) favoured the for
mation of only one system of (A) type.
The divalent T M P D cation present in (C) is most likely stabilized by a weak
anion of mannitol hexanitrate. This is contrary to the existing statement [10] of
its low stability due to the presence of the strong perchlorate anion.
However, we were unable to isolate the solid complex as it readily decomposes.
Infrared
spectra
We were able to confirm the presence of divalent T M P D cation (in В, B, and D
system) by infrared spectroscopy. M H N and T M P D were dissolved in the equimolar proportion in 1,2-dichloroethane, or benzene, or acetonitrile and the spectra
(Fig. 5) were taken 24 hours later.
Band at 1620 c m - '
The most important feature of the spectrum of the complex is a strong band
at 1620 c m absent in the spectra of components. The band should be assigned
to the C = N bond. According to the literature [8] the band at 1630 c m in the
spectrum of ^^A^'^Vtetramethyl-^-quinonediimonium diperchlorate in potassium
bromide should be assigned to this bond.
- 1
- 1
Bands at 1540 and 1640 c m
- 1
and shoulder at 1520 c m "
1
The bands should be assigned to the selected vibrations of C = C in the quinoid
ring.
D-Mannitol
Hexanitrate
as Electron
Acceptor
in CT
Complexes
409
Bands of the O-nitro groups
- 1
2 6
The band at 1680 c m , which is one of three О — N 0 bands present in M H N ,
was subjected to a bathochromic shifting by 20 c m in our complex. Such shifting
is not unusual in CT complexes [10].
2
- 1
Ja
e so
£
in
J
60
•
\
40
20
з
—
80
i
li
60
-
40
iw
\
У V
20
•-л
v
Fig. 5. Infra red absorption spectra of solutions in ben
zene; A — M H N ; Б — T M P D ; С — M H N + T M P D
(mole ratio 1:1) (solid line) Tetramethyl-/?-quinonediimonium diperchlorate broken line
s-
r _.
ш
и
ШЮ0
\1600
-
\
и
—
1
*Л
a
j-
f
B0O
Ш
Wavenumber
1
Another band in the complexes has the frequency 1360 c m - . The band is not
present in the components. It occurs in the region of inorganic nitrate ion ( O N 0 )
[11]. We suggest that the band in question should be assigned to O N 0 groups
in M H N negatively charged under the influence of the electron donor of a low ioni
sation potential.
_
2
2
Experimental
The solvents: benzene, methanol, n-heptane used were pure for spectroscopy grade.
1,2-dichloroethane was purified as described in our previous paper [1].
Acetonitrile was purified by repeated refiuxing over P 0 and distilling [15].
D-Mannitol hexanitrate was prepared and purified as in our previous paper [1].
Electronic spectra were examined on a Unicam SP. 500 spectrophotometer with thermostated
cells of 0.2 cm thickness of the liquid layer and concentration с = 0.002 mol/1.
Infrared spectra were measured on a Unicam SP. 200 spectrophotometer in an N a C l cell.
The thickness of the layer was 0.2 mm. Concentration of the substances (in 1,2-dichloroethane,
benzene, and acetonitrile) was 0.05—0.02 mol/1.
In order to have a detailed examination of the bands, a Hilger H-800 spectrophotometer was
also used. The frequencies reported in our present work were determined on a Hilger H-800 appa
ratus.
2
5
T. U r b a ń s k i et al.
410
Electric resistance
н-Heptane (10 ml) was added to a solution of M H N and T M P D in acetonitrile 20 ml con
centration of each of the components was 0.05 mol/1.
The mixture was shaken for 15 min., н-heptane layer was separated, diluted with 1,2-dichloroethane in proportion 1:1 (by vol.). Different quantities of methanol were added to the heptane —
dichloroethade solution. The conductivity was measured by means of a "Eureka" 37/62 conductometer.
D E P A R T M E N T O F O R G A N I C C H E M I S T R Y , T E C H N I C A L U N I V E R S I T Y , W A R S A W , K O S Z Y K O W A 75
( K A T E D R A T E C H N O L O G I I O R G A N I C Z N E J II, W A R S Z A W A )
INSTITUTE O F O R G A N I C C H E M I S T R Y , POLISH A C A D E M Y O F SCIENCES, W A R S A W 42, K A S P R Z A K A 44/52
( I N S T Y T U T C H E M I I O R G A N I C Z N E J PAN)
REFERENCES
[1] В. H e t n a r s k i , W. P o ł u d n i k i e w i c z , T. U r b a ń s k i , Tetrahedron Lett., 1970, 3.
[2]
,
, Bull. Acad. Polon. Sci. Sér. Sci.
Chim., [see preceeding paper in this issue],
[3] T. U r b a ń s k i , Roczniki Chem., 13 (1933), 399; 14 (1934), 925; 15 (1935), 191 ; 16 (1936),
359; 17 (1937), 474.
[4] А. С. A l b r e c h t , W. T. S i m p s o n , J. A m . Chem. S o c , 77 (1955), 4454.
[5] P. J o b , Compt. Rend., 180 (1925), 928; A n n . Chim. Phys., (10) 9 (1928), 113.
[6] G . B r i e g l e b , Elekronen-Donator-Acceptor-Komplexe, Springer, Berlin, 1961.
[7] S. D a h n e , Z . Chem., 3 (1963), 191.
[8] F . R i t s c h l , Spectrochim. Acta 23A (1967), 655.
[9] T. U r b a ń s k i , M . W i t a n o w s k i , Trans. Faraday S o c , 59 (1963), 1047.
[10] J. R o s e , Molecular complexes, Pergamon Press, Oxford, 1967.
[11] A . D . C r o s s , Introduction to practical infrared spectroscopy, Butterworth, London, 1967.
[12] A . W e i s s b e r g e r , E . P r o s k a u e r , D . R i d d i c k , E . T o o p s Jr., Organic solvents, Intersci.,
N . Y . , 1955.