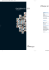the Scanned PDF - Mineralogical Society of America
Transcription
the Scanned PDF - Mineralogical Society of America
THn AUERICAN MIxERALocIST
JOURNAL OF THE MINERALOGICAL
Vol. 51
ZELLERITE
SOCIETY OF AMERICA
NOVEMBER-DECEMBEIT, 1966
AND NIETAZELLERITE,
CARBONATESI
Nos. 11 and 12
NEW URANYL
R. G. Cor-nuaN,D. R. Ross aNl R. Mnvnowrrz,
Geological
Survey, Menlo Park, Cali.f.;Washington, D. CtI . S.
Agsrnecr
'I'he
mineral zellerite, named after Howard D . Zeller, occurs at the Lucky Mc uranium
mine, Fremont County, Wyoming, as pin cushion clumps of needlelike orthorhombic
crystals on oxidized uranium ore and is intimately associated with gypsum and secondary
iron oxides. The recalculated analysis gives CaO 10 3, UO3 53'9, COr 16.9, HrO+ (110')
12.3,irj'2o- (110') 6.6 after SiOz*insoluble 0.5, R:Or 1.0, KeO 0.2, NarO 0.4 were sub'fhis
tractedtas impurities. This gives a ratio of CaO: UOr: COgHTOof 1.00: 1.03: 2 09: 5.81'
formula
yields
CaUO:(COs):
the
content,
regarding
the
rvater
and
other
considerations
ratio
.5HrO for the fully hydrated mineral On partial dehydration, a probable metacalcium
uranyl dicarbonate of formula CaUOr(CO:):'3HuO, metazellerite, is produced.
Single-crystal rc-raystudies of both hydrated and dehydrated material yield: (1) zellerite, ortlorhombic, probabl" spacegroup Pmn2ror Pmnm, a 11 220,b 19.252,c4.933 A, cell
cont€nts 4[CaUO:(CO3):.sH:O], cell volume 1065.4 Aa; (2) metazellerite, orthorhombic,
space group Pbn2r or Pbnm, a9.718, b 18.226, c 4'965 A, cell contents 4[CaUOz(COr):
3HrOl, cell volume 879'5 A3.
The fully hydrated mineral is lemon yellow with a duil luster and no cleavage. Measured specific gravity is 3.25; calculated 3.24 gm/cm}. The optical properties are: a 1.536,
pleochroism:
Z:c, dispersion weak vlrl
P 1.559, 7 1.697, biaxial (*), 2V:30-40',
z:light yellow, If :colorless, X:colorless. The mineral has a weak patchy green fluorescence under Iong and short ultraviolet light. It forms as an oxidation product of uraninitecoffinite in the weathering zone) where Pco, is greater than that in the atmosphere and pH
is greater than 7.
INrnooucrrou
studies on the uranium minerals making up the oresfrom the Gas Hills
deposit in Wyoming have revealed a complex suite of secondaryminerals
in the oxidized zone. In July 1955, Howard D. Zeller, U. S. Geological
Survey, discoveredan unknown yellow, fibrous uranium mineral in the
oxidized zorre of the Lucky llc mine. Preliminary tr-ray studies by
Daphne R. Ross indicated that the mineral has a structure distinct and
different from previously describedsecondaryuranium minerals. Chemical analysis confirmed that it is a uranyl carbonate of distinct composi1 Publication authorized by the Director, U' S. Geological Survey.
t567
1568
R. G. COLEMAN, D. R. ROSS,lN'
R. X,TEYROIYITZ
tion. The mineral, a calcium uranyl carbonate, CaUOz(CO)r.5HrO, is
named zellerite alter Zeller. N{uch of the preliminary geologic investigation on these uranium depositscarried out by him were instrumental in
the iater successfulexploration and mining operationsof this important
urairium district (LeIler,1957;ZeIIeret a|,.,1956).The nameszelleriteand
metazelleritehave been approved by the New Mineral Names Committee, IIIA.
OccunnnNcB
Zc|Ierit-eis found as incrustartionson arkosic country rock; it forms
during slrrfaceoxida,tionof the uranium ore. The unoxidized ore in the
Luckr' \Ic mine is a fine-grainedmixture of uraninite, coffinite,and iron
sulfidesfiliing intergranular areasin arkose.The coarseand porous nature of the host rock allows rapid moist-nir oxidation of these primary
uranium minerals :rnd sulfides.In the early stagesof oxidation, black
primary ore commonly contains fine seamsof green liebigite ICar(UOr)
(C()3)3'10FIzO]intergrown with gypsum. Ilimming this dark ore is a rusty
brolvn zone where the oxidartionhas effectedthe breakdown of all the
primarl- nranium minerals and someof the iron sulfides.Zelleriteis most
commonlv present in this zone where it is associatedwith gypsum,
limonite and partialiy altered iron sulfides.It forms extremelyfine fibers
that aggregateinto pincushion clumps (Fig. 1). Schoepite [UOz(OH),
.HzO], meta-antunite
I C a ( U O z ) r ( P O D z . 2 .65. 5 H r O ] , u r a n o p h a n e I C a
(LTOz)zSLOr'6HrO],
and uraniferousopal are presentin the more intensely
oxidizedzonescontiguousto the zellerite.
A secondoccurrenceof zelleritein Wyoming from the Pat No. 8 mine,
Powder River Basin, Wyo., was brought to the attention of the senior
author by William Sharp (ln Sharp and Gibbons, 1964),1J.S. Geological
Surve1.,and a third occurrence in New X,Iexicohas been confirmed by
Granger (1963). These occurrencesare similar to that at the Lucky N{c
mine in that the zellerite forms as small veins and clumps in oxidized
zonessurrounding the unoxidizedore.
Pnoponrrrs
Physical. Zellerite crystals are so fine that indivudual crystal faces could
not be discerned.The habit of these crystals was always found to be
fibrous and hairlike, aggregatinginto pincushion clumps (Fig. 1). The
mineral is quite soft, with a hardnesssimilar to gypsum. Cleavagewas
not observed; however, the aggregatedclumps always fracture in the
d i r e c ti o n o f e l o n g aito n .
The measuredspecificgravity of the mineral is 3.25*0.01 as determined by suspensionin a mixture of bromoform and methylene iodide.
NEilT LlRANYL CARBONATES
1569
The variation in density noted among the individual specimenswas attributed to differencesin state of hydration.
Zellerite is a light lemon yellow and on dehydration assumesa chalky
yellow color. fn long and short wave ultra-violet light, zelleriteshows a
weak patchy green fluorescence;however, as compared with other
uranium carbonates,particularly liebigite, it is practically nonfluorescent.
opticat,.In ordinary white transmitted light, zelleriteis transparent and
light yellow. Individual crystals seld.omexceed2 mm in length and 5p in
Frc. 1. Zellerite showing crystalline habit. The pin-cushion clumps are
implanted on gypsum. N4.5.
width. variations in indices were noted and could be related to the
changesin the hydration state of the mineral. Indices ol the lower hydrate were not measuredbecausesuitablecrystalscould not be obtained.
The optical constants of zellerite given in Table 1 are consideredto characrcri)e the fully hydrated zellerite. Using the Gladstone-Dalelaw (Jaffe,
1956), the averageindex calculated for zellerite comparesfavorably with
the average measured index.
Chemistry. The one-gram sample used for analysis was carefully purified
by centrifuging in organic heavy liquids' The final concentrate was con-
1 57 0
R. G, COLEMAN, D. R ROSS /N'
R. MEYROI,I.ITZ
sideredto be at least 98 per cent pure; the remaining 2 per cent includes
minor amounts of clay, quartz and limonite. The metastability of zellerite meansthat even though the final separatewas pure, somemetazellerite was present and no precautions were taken to control the humidity.
Spectrographic analysis and r-rav studies of the concentrate confirm the
optical estimate of sample purity.
Proceduresof the chemical analysis are described briefly. The original
t^""rt."-"*"-:,M
Zellerite
Crystal system
Space Group
b
c
Celi volume
Cell contents (Ideal
formula)
Density (calc. for
ideal formula)
Specific gravity
measured
Biaxial negative
d colorless
B colorless
7 light yellow
Orientation
Dispersion
n average (measured)
a calculated2
Orthorhombic
Pmn2t or Pmnm (probable)
Powder datar Single crystal
11.220+0.01s1
41.14A
1 9 . 2 5 2 + 0 1 6A 1 9 . 1A
5
4 933t0.0164 4.s4A
1 0 6 5 . 4 + 2A. 3
4[CaUOz(COs)2.5HzO]
3.24,
Metazellerite
Orthorhombic
Pbn2r or Pbnm
Powder datal Single crystal
9.718+0.0A
0 5 9 . 7 5L
1 8 . 2 2 6 + 0 . 0A
0 9 1 8 . 2A
4 . 9 6 5 + 0 . 0 0 4 4+ S r A
8 7 e . s + 0 .A7
4[CaUOz(COr)z'3HzO]
3.414
3.25+0.01
2V:30-40"
1.536+.005
+ .005
1. 5 5 9
1 697+.005
Z:C
tt) r
1.597
1 595
2V?
Z:C
?
1.626
1 Data derived from least-squares
refinement oI r ray powder data listed in Table 3
using the method of Evans et al. (1963\ .
2 Calculated using the Gladstone-Dale
law (Jatre, 1956).
sample was air dried to a constant weight at 110'+soc. The resulting
loss in weight is reported as minus water. However, part of this minus
water is thor,rghtto be essentialto the mineral in order that there be 20
moleculesof HzO per unit cell. A modified microcombustion train of the
type made for the determinationof carbon and hydrogenin organiccompounds was used to determine HzO+ and COz. For this, a 20-mg sample
was decomposedby ignition at 900"C in a stream of oxygen.
The Rror was determinedby differenceafter the total Rzor*uros had
been determined gravimetrically using carbon dioxide-free NHaOH and
r57|
NEW URANYL CARBONATES
methyl red as the indicator. UOg was determined spectrophotometrically
by the ammonium thiocyanate procedure in acetone-watermedium after
solutionof the RsOaresiduein (1*1) HNOg.
CaO was determined as the sulfate after it was separatedfrom an 85 f
per cent ethyl alcohol solution. Qualitative spectrographicanalysis of the
CaSOaprecipitate showedthat the only major constituent was calcium;
there were no minor constituents.
KzO and NarO were determined by flame photometry. The filtrate
from the CaO determination was transferred to a platinum evaporating
dish, and taken to dryness, then ignited. The remaining salts were disTLlr,e 2. Cneurcnr- ANervsrs ol Znr-lrnrte
Analyst, Robert Meyrowitz
CaO
UOa
COz
HgO+
SiOz*inso1.
RzOs
KrO
Na2O
Total
HrO(-)
1 0 .8
56.6
1 3. 0
0.5
1.0
0.2
0.4
100.2
6.9
10.3
5 3. 9
16.9
12.3
.1836
.1884
.3840
.6833
1. 0 0
1. 0 3
209
3.72
6.6
.3833
2.09
100.0
1.
2.
3.
4.
Weight percent of the oxides determined on sample air dried to 110'C.
Recalculation of analyses to 100/6 without SiO2, RzOa,KzO, NazO and insolubles.
Molecular proportions.
Molecular ratios.
solved in (1*1) HCI and transferred to a volumetric flask and diluted to
volume. Aliquots of this solution were used for the KrO and NasO determinations, comparing them to standard potassium and sodium solutions.
Twenty-five milligrams were usedfor the HrO(-), SiOz*insoluble RsOa,
CaO, UO3, KzO, and NazO determinations.
The recalculated analysis of zellerite (Table 2) producesa formula that
is distinct from the uranyl carbonatespreviously described.Assumingthat
part of the HzO- combined with H2O+ characterizesfully hydrated zellerite, the calculated formula is CaUOz(CO3)r'sHrO.Variation in the
measured physical constants indicates different hydration states of zellerite. If we assumethat H2O- as determined is easily lost, then a lower
would becomestable at higher
hydrate metazelleriteCaUOr(COr)2.3H2O
1572
R. G. COLEMAN, D. R. ROSS.4N' R. MEYROWITZ
temperatures or lower humidities. N{etazellerite was not available in
amounts sufficient to obtain precisephysical measurementsrelated to the
changes accompanying hydration-dehydration, but as is demonstrated
by the r-ray results,the structural changeon dehydration is distinct and
can be characterized.
X-ray crystallography.The r-ray powder pattern made of the fresh ,,zellerite sample" showed only the fully hydrated phase, zellerite,to be present (Table 3). Powder patterns made subsequently,after the sample
had been exposed to the laboratory atmosphere for several weeks, invariably showedthe dehydrated phase,metazellerite,to be present with
zellerite.one r-ray powder pattern (Table 3) showed almost total conversion of zellerite to metazellerite.
Several needlelike crystals from the "zellerite sample', were examined
with the Buerger r-ray precessioncamera. Invariably a strong singlecrystal pattern of the dehydrated metazellerite phase would appear in
parallel orientation with the weaker single-crystal pattern of the fully
hydrated zellerite phase. zellerite is apparently unstable with respect to
metazellerite under the conditions of the r-ray experiment, although it
may persistmetastably with metazelleritefor long periodsof time. Buerger precessionphotographs, exposedfor 100 hours with molybdenum zirconium-filtered r-radiation, were made of the \kl, Ikl, h\l, hlt and,h2l reciprocal lattice nets. These photographsshow both phasesto be orthorhombic with the following unit-cell data: zellerite,a ll.la, b 19.15,c 4.9a
A, probablediffraction symbol P mn* ;metazellerite,a 9.76,b 18.2,c 4.9a ft,
diffraction symbol Pbn*. Owing to the weaknessof the r-ray pattern, the
diffraction symbol of zellerite cannot be positively assigned.
fn order to verify the unit-cell parameters obtained from the rather
poor single-crystal patterns and also to obtain more accurate values for
theseparameters,the r-ray powder data of both phaseswas subjectedto
Ieast-squares
refinement(Evans et a|,.,1963)using the single-crystaldata
as the starting parameters.The final parametersas obtained from the
Ieast-squaresanalysis are given in Table 1 along with the other pertinenl
crystallographic and optical data.
The dehydration of zellerite to metazellerite involves a decreasein cell
volume of 186 43. This volume changestronglv suggestsa loss of 8 or 10
HzO molecules.The spacegroup requiresthat there be an even number of
atoms of each type in the unit cell. Therefore zellerite CaUOr(COa)z
' 5HzO, with Z:4 probably has two more HrO moleculesper unit formula
than metazellerite,CaUOr(COr)2.3H2O,with Z:4 if it is assumedthere
is a lossof 8HzO molecules.Should we presumea 10HzOlosson dehydration, the formula would have to be written CaE(UOz)+(COr)s.20HrO,
with
TanrB 3. X-R.rv DmlnncrroN
Pownrr
Dlra
ron Zstr;Enlrn
Zellerite
Calculatedl
dhkL
hht
010
110
o20
120
030
200
130
2ro
220
040
011
101
140
111
02r
230
t2l
031
050
131
310
240
150
211
320
221
041
330
060
231
250
160
051
301
340
311
241
151
321
400
260
410
070
33r
420
061
350
170
25t
161
430
270
oo2
ot2
360
261
440
1 9. 2 5
969
963
731
642
5 610
5.570
5.386
4 847
4.813
4 778
4 515
4 .423
4.396
4 390
4.224
4.088
3 911
3 850
3 693
3.671
3 653
3 642
3.638
3 486
3.457
3.445
3.293
3.23r
3 209
3 208
3 085
3.035
2.980
2.953
2.945
935
930
847
805
Metazellerite
Observed,
,thkt
I
966
7 33*
5 591
5.405x
4.848*
4 407
4 2404
3 651
3 463+
3 232*
2.947
2 851*
2.718
2.687
2.5324
2.468
2.433*
2.425
2.423
Calculatedr
thkt
hkt
o20
I 113
110
120
130
200
2to
040
101
02l
111
220
140
r2l
230
131
150
2t1
041
240
221
310
141
320
060
231
250
160
330
8.57.5
6 648
5 152
4.859
4 695
4 557
4.422
4.360
4 297
4 288
4 1.26
3 978
3 795
3 575
3.413
3.411
Observed2
dht
9.10*
I
100
100
241
301
31t
340
321
061
260
170
25r
r61
o02
33r
400
350
410
022
tt2
420
34r
122
270
261
080
430
t7l
3 323
3 245
3.189
3 173
3 052
3.038
3 015
2.916
2.899
2.858
2 813
2.762
2.713
2 683
2 640
2.600
2 59t
2.576
2 515
2 51+
2.504
2.483
2 477
2 430
2.421
2 408
2.395
2.384
2 348
2 331
2 326
2.295
2.286
2.278
2 256
2.244
180
360
202
2.2s7
2.218
2.216
2 211
151
/ 6.)
2 716
2 750
2 703
2 693
2 690
2 683
2 67r
2 670
2 616
2 570
2 534
2.46e1
2 466J
2.446
aNo Mrr,nzrllrnttE
5 140*
4 868*
4.695*
4 552*
4.4124
2
13
36
18
18
4 296
36
3 978*
3.794*
3 579*
18
50
6
3411
2
3 330*
13
3.173*
18
3.033+
9
2.91-(*
2
2 858*
2 8t2*
2 763x
4
2
2
2 6874
2.639*
6
1
2.580*
2
2.508*
6
2 478*
18
2.415
2 -344*
2.294*
2.257*
2.2t9
Tl^nrn 3-(Continud.)
Zelleite
Calculatedr
dhht
hkt
411
080
07l
tt2
o22
421
351
180
171
t22
o32
431
450
202
132
212
510
370
280
271
222
o42
520
361
441
081
142
090
232
181
530
460
190
052
451
312
501
152
540
511
380
371
281
322
2 419
2.406
2.402
2.390
2.389
2.364
2 357
2 353
2.349
2.337
2.302
2.279
2.267
2.258
2 255
2 242
2.229
2.216\
a
ol1f
208
198
195
2.185
2.184
2 r75
2 163
2.154
2.139
2 130
2.124
2 1,18
2 1r2
2 101
2.O77
2.060
2.O47
2 041
2 043
2 042
2.O34
2 031
2 024
2 021
2 018
2 0r3
Metazellerite
Observed2
dhil
I
Calculatedl
dhkt
hkt
212
o42
351
411
222
440
2 359
2 263
2.214
2 173*
2 138t
1L)
421
271
232
081
280
431
370
181
361
450
152
2.t95
2 .1 8 0 1
2 167
2 149
2.144
2 .r 2 7
2.r22
2.083\
2.o78J
2 071
2 063
2 054
2 029
r nr<l
2.o241
2 022
2.008
Observed2
dhkt
I
2.178
2 147*
2.080
2.O25
18
1. 9 8
193
1. 9 0
1. 8 7
1. 8 4
r.82
1.78
t.73
1.71
168
1.67
r.62
160
|.57
1.56_
I
3
1
9
2
2
6
o
1
6
2
2.103x
2.057*
2.O23
194
1. 8 6
1. 8 4
182
179
1.73
1 7l
164
r d-spacings were calculated from the unit-cell parameters listed in Table 1. These parametere were obtained from a least squares refinement of the observed d-spacingsmarked with an-asterisk in the table above.
2 Zellerite from Lucky Mc mine, Wyo CuKa radiation, Ni filter (X:1.5418 A) Cameradiameter:114.59
mm. Lower limit 2a measurable: approximately 8' (11.0 A). Intensities measured with a calibrated intensity
strip.
NEW URANYL CARBONATES
l.) /.)
Z : l , I o r z e l l e r i t ea n d C a a ( U O r ) (nC O r ) r . 1 0 H r O ,w i t h Z : 1 , f o r m e t a z e l Ierite. The chemical analysiswould suggestthat an averageof 20 HzO
moleculesper cell is correct for zellerite and observedchangein volume
would indicate that I2H2O moleculesper unit cell for metazellerite is correct.
To test the validity of the formula for zellerite,CaUOz(COa)z.5HzO,
we have a calculateddensity of 3.242 g/cmt, which comparesfavorably
with the measuredspecificgravity of 3.25+0.01. The calculatedaverage
refractive index (Jaffe, 1956) of zellerite,using the ideal formula and calculated density from the r-ray data, is 1.595, and the average of the
measured values on the analyzed material is 1.597+0.005. The close
agreement between the measured and calculated values for density and
refractive index substantiates the proposed formula for zellerite. Dehydration of zellerite probably involves loss of 8 or 10HrO molecules,but
verification of the metazellerite formula awaits further optical and specific gravity measurementson this lower hydrate.
Relationshipto otherwranyl carbonates.Zellerite is the only known natural
representative of the uranyl dicarbonates having the general formula
M'+(UOz)(COs)z'nHzO.Bayleyite, swartziLe,liebigite, the more comTrnr,u4. Coup.tnrsoN
ol Cer-cruueNnMecNnsruuUnexvr.CansoxA,rns
Zellente
Liebigiter
Bayleyite,
Swartzirel
^*:Po'l::L
Ca(Uocrcoa)zcar(Uoz)(cor)rMg:1U^o)r1Coa)r',1';ii.',';;;]-caMs(Uo,),':;;H;,'
.Suzo
.10Hro
.18Hro
(coa)s.12Hro
a
P
1.536 light
yellorv
| 559 colorless
.y
| 597 colorless
Color
Opticsign
2V
Density
Flourescence
Lemon yellow
(+)
30-40.
3 2510 01
Week, patchy
green
Long and sbort
wave
Fibrous
Habit
Crystal system
Spacegroup
a
b
c
p
1 497 colorless
1 455 pinkish?
1.502 colorless
1.465 colorless
1 502 pale
greenish yellow
1 .539pale
greenish yellow
Sisl<ingreen
(+)
40"
2 4t
Brrght green
1 .490 pale
yellow
1 500 pale
yellow
Yellow
(-)
30.
2 os
Weak, color
uncertain
Long and short
wave
Prismatic
acicular
Monoclinic
Ph/a
2 6 . 6 . 5A
15.31
6.53
93'04'
1 . 508 colorless
1 5l yellow
1 .525 colorless
1 . 54 yellow
pale green
(+)
?
2.57
Cream yellow
Short wave
Green
(-)
40.
2.3
Bright yellowisb
gfeen
Short wave
prismatic
prismatic
Long and short
wave
Prismatic
Orthorhombic
Orthorhombic
Pnn2t or PNnm
Bboz^
1 1 . 2 2 o| t
1 6 . 7 1A
l9 252
17 55
4 933
13 79
-
t Data from Frondell (1958).
acicular
Monoclinic
3 2 . 6i \
23.8
9 45
90.
Monoclinic
p2t/n or p2t
11.t2L
14 72
6-i4
s9"26'
1576
R. G. COLEMAI{, '. R. ROSSAND R. MEYROII.ITZ
monly reported uranyl tricarbonates,have the ratio of 1:3 for (UO):
(COr) (Table 4) . Rutherfordine, the anhydrous uranvl carbonate,
UOzCOr,could be consideredthe end member of the uranyl carbonatesif
we considera seriesas shown in Fig. 2.
The low-temperaturechemicalrelationshipsin the U-O:-HrO-COrslstem have been exploredadequately(Hostetler and Garrels,1962; Garrels
and Christ, 1959)so that the natural occurrenceof uranyl carbonatescan
be explained.All of theseauthors have pointed out that both uranyl tri-
Liebigile
lOHZo
Co2(uO2)-(CO3)3'
Co(uO2XCO3)2'5H20
Z e ll e r i t e
+2
UO
S c h o e piie
U O 2 ( O H ) 32' HO
92
Frc. 2. Ternary diagram showing some mineral phases in the system Ca2+-CO32--UO'2*
given in molecular per cent.
carbonate [UOr(CO3)s]4-and uranyl dicarbonate [UOz(COr)z'2Hrg1zcomplexesmay form in waters with pcor) 10-3 atmospheresand total
ionic activity in excessof 10-6.As one would suspect,the activities of the
uranyl carbonate complexesare very sensitive to variations in pco, and
pH of the containing solutions. The Eh-pco, diagrams constructed by
Hostetler and Garrels (1962,p. 144-148)illustrate the stability fields of
the uranyl carbonatecomplexesin responseto changing conditions.A1though these diagrams are representativeof conditions at 25oC and 1
atmosphere, the environment of oxidation of the Lucky X'Ic uranium ore
is so close to the depicted conditions that the natural occurrenceof the
uranyl carbonates can be related to the diagrams. Rutherfordine oc-
NEI\/ URANYL CARBONATES
t577
cupies a narrow field of stability with solutions whose pH is about 6,
under oxidizing conditions, and with a total dissolveduranium activity in
excessof 10 a. When the partial pressureof COzis near that of the atmosphere(10 t.r atmospheres),both schoepite(UOz(OH)z'HzO)and rutherfordine (UOrCO3),are stabletogether (Garrels,1957).Schoepitebecomes
unstable with an increaseof pco, in accordancewith the reaction
UOr(OH)r.HrO
f COz+ UOsCOs
+ 2H2O;
should the increaseof pco, be accompaniedby increasein pH, the uranyl
dicarbonate complex becomes stable and those solutions containing
enoughCa2+will precipitatezelleriteon evaporation.By further increasing the pH at the same activities, the uranl,l tricarbonate complex becomesstable instead of the dicarbonate,and solutions of this composition on evaporationwould precipitate liebigite.
As stated earlier, Iiebigite forms as veins and incrustations in the primary dark ore during the early stagesof oxidation at the Lucky Mc mine
and before the breakdown of pyrite. Once the pH of the oxidizing surface
is Ioweredby the pyrite alteration,zelleriteis precipitatedfrom thesesolutions rather than liebigite. In those areas removed from primary ore
where COzhas been depleted,schoepiteforms in associationwith uranyl
silicates. Zellerite therefore must occupy a field of stability intermediate
between rutherfordine and liebigite in the zone of weathering.
AcxuowrnocMENTS
We would like to thank HowardD. Zeller for bringing samplesof this
mineral to us for study and for his many helpful suggestionsregarding its
occurrence.Malcolm Ross was instrumental in finalizing the interpretation of the *-ray data and Charles Christ provided many helpful suggestions that improved the manuscript. Terry Clark carried out many of
the laboratory measurementson the physical properties.
RntnnnNcns
(1963) The least squares reEvars, H. T., Jn., D. E. Arpr.eueN aro D. S. HaNowlmrn
finement of crystal unit cells with powder diffraction data by an automatic computer
indexing method. Am. Crgstal Assoc Program Ann. Meet.,1963,pp.42-43 [abs.E-10].
Fnononr, C. (1958) Systematic mineralogy of uranium and thorium. U. S. Geol'.Surtey
BulI.1O64, l-4OO.
Gennnrs, R. M. (1957) Some free-energyvalues from geologic relations. Am. Mineral'.42,
780-79r.
-
AND C. L. Cnnrsr (1959) Behavior of uranium minerals during oxidation. U. S.
Geol. Swtey ProJ. Paper 320, pt. 6, 81 89.
GneNcnn, H. G. (1963) Mineralogy. In, "Geology and technology of the Grants uranium
region." New Meni,coBw. Mi,nes Mineral ResourcesMem.15,2l-37.
Hostutmn,
P. B. ,wn R. M. Geaanr-s (1962) Transportation and precipitation of ura-
1578
R, G. COLEMAN,
'.
R. ROSS AND
R. MEYRO\AITZ
nium and vanadium at low temperatures with special reference to sandstone-t)?e
uranium deposits. Econ. Geol. 57, 137-L67.
Jmm, H. W. (1S56) Application of the rule of Gladstone and Dale to minerals. Am. Mi,ne r a l , . 4 L , 7 5 77 7 7 .
Sumr, W. N. .tNo A. B. GreeoNs (1964) Geology and uranium deposits of the southern
part of the Powder River Basin, Wyoming. U. S. Geol Suney Bul,l,. 1147-D, 1-60.
Znt:,ar., H. D. (1957) The Gas Hills uranium district and some probable controls for ore
deposition. Wyoming Geol,.Assoe. Guidebooh, 12th Ann. Fielil ConJ., Sept. 1957, pp.
156-160.
-,
P. E. Sorsrnn nNo lI. J. HvorN (1956) Preliminary geologic map of the Gas Hills
uranium district, Fremont and Natrona Counties, Wyoming. U, S. Geol. Suney Mine.ral,.Inr. Map. MF-83
March 10, 1966; acceptedf or publi.cati.on,March 28, 1966.
Manuscript receit:ed,,