Radical surgery for gallbladder cancer: current options 1
Transcription
Radical surgery for gallbladder cancer: current options 1
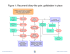
European Journal of Surgical Oncology 2000; 26: 438–443 doi:10.1053/ejso.1999.0918, available online at http://www.idealibrary.com on Radical surgery for gallbladder cancer: current options A. Muratore, R. Polastri and L. Capussotti 1° Department of Surgery, Ospedale Mauriziano ‘Umberto I’, Torino, Italy Gallbladder carcinoma is the most common malignancy of the biliary tract. There are still many controversies regarding the type of curative surgical treatment for each stage of the disease. The staging system used is the TNM classification of the International Union Against Cancer. Different patterns of spread characterize gallbladder cancer but the two main types are direct invasion and lymph node metastases; since only the depth of invasion can be easily recognized by imaging techniques, it becomes the main variable in choosing the appropriate surgical treatment. Most Tis and T1 tumours are incidentally discovered after cholecystectomy for cholelithiasis and no further therapy is requested; for pT1b tumours, relaparotomy with hepatic resection and N1 dissection is associated with a better survival. For T2 tumours, cholecystectomy with hepatic resection and dissection of N1–2 lymph nodes is the standard treatment, with a 5-year survival of 60–80%. The only chance of long-term survival for patients with a T3–T4 tumour is an extended operation combining an hepatic resection with an N1–2 dissection with or without excision of the common bile duct. A subset of patients with peripancreatic positive nodes or invasion of adjacent organs seems to benefit from a synchronous pancreaticoduodenectomy. 2000 Harcourt Publishers Ltd Key words: gallbladder; surgery; treatment; laparoscopy; lymph nodes. Introduction Carcinoma of the gallbladder was described for the first time by Maximilian Destoll in 1777 but the first liver resection for this disease was performed by Keen in 1881.1,2 More than 80% of gallbladder cancers are adenocarcinomas; this malignancy is the most common of the biliary tract and represented approximately 8.4% of the estimated cases of liver and biliary tract cancers diagnosed in the USA during 1994–1995.3 Carcinoma of the gallbladder is an aggressive and late symptomatic disease and most patients are treated at an advanced stage with a poor prognosis. During recent years, extended operations combining a resection of the liver with wide lymph node dissection have improved the long-term survival.5,6 However, there are still many controversies regarding the type of surgical treatment for each stage of the disease.3,4,7 Staging system The old classification of Nevin has been used for many years;8 there are five stages defined according to the parietal extension of the tumour: Stage I: intramucosa only; Stage II: mucosa and muscularis; Stage III: mucosa, muscularis Correspondence to: Andrea Muratore, V. Nizza 39, 10100 Torino (TO), Italy. Tel: +44 (0) 141-910124; E-mail: andreamurat@hotmail.com 0748–7983/00/050438+06 $35.00/0 and subserosa; Stage IV: cystic lymph node plus all three layers; Stage V: liver by direct infiltration or metastases (to the liver or any other organ). The most used staging system for gallbladder carcinoma is the TNM classification of the International Union Against Cancer (UICC)9 (Table 1). Pattern of spread Cancer of the gallbladder is characterized by different patterns of spread; an accurate knowledge of them is important for determining the most rational surgical treatment: (1) Direct invasion of adjacent organs is a common mode of spread: in terms of frequency, about 60% of the patients have infiltration of the liver, followed by the colon, bile duct, duodenum and pancreas.10 Macroscopically, there are two patterns of hepatic invasion (Fig. 1): liver-bed type, in which the tumour extends directly into segment IV–V from the liver bed; and hilum-type, in which the cancer tends to extend toward the hilum and to invade deeply into the liver along Glisson’s sheat.1 When the type of invasion is studied histologically, most liver-bed carcinomas have an expansive spread, whereas most of the hilum type carcinomas have an infiltrative spread.2,13 The knowledge of these two different patterns of infiltration is clinically important for determining the resection procedure: resection of segment IVb–V in patients with liver-bed type carcinoma achieves a high rate of negative margins and seems associated to a better survival than wedge resection; in the hilum type, extended right 2000 Harcourt Publishers Ltd Radical surgery for gallbladder cancer 439 Table 1. TNM classification of gallbladder cancer9 T-Primary Tumour Tx primary tumour can not be assessed T0 no evidence of primary tumour Tis carcinoma in situ T1 tumour invades lamina propria (T1a) or muscle layer (T1b) T2 tumour invades perimuscular connective tissue without extension beyond serosa or into liver T3 tumour perforates serosa (visceral peritoneum) or directly invades into one adjacent organ or both (extension into the liver Ζ2 cm) T4 tumour extends >2 cm into the liver and/or into two or more adjacent organs (stomach, duodenum, colon, pancreas, omentum, extrahepatic bile ducts, any involvement of liver) N-Regional Lymph Nodes Nx regional lymph nodes can not be assessed N0 no regional lymph node metastasis N1 metastasis in cystic duct, pericholedochal and/or hilar lymph nodes (hepatoduodenal ligament) N2 metastasis in peripancreatic (head only), periduodenal, periportal, common hepatic artery, coeliac and/or superior mesenteric artery lymph nodes M-Distant metastasis Mx distant metastasis can not be assessed M0 no distant metastasis M1 distant metastasis Stage Grouping Stage 0 Stage I Stage II Stage III Stage IVA Stage IVB Tis T1 T2 T1–2 T3 T4 Tany Tany N0 N0 N0 N1 N0–1 N0–1 N2 Nany M0 M0 M0 M0 M0 M0 M0 M1 Fig. 1. Type of liver invasion in gallbladder cancer. A: liver-bed type; B: hilum type. hepatectomy achieves a higher rate of negative margins than resection of segment IVb–V, but the type of resection does not seem to improve the survival.12 (2) Many studies have stressed the significance of haematogenous hepatic spread by a variable number of small veins of the gallbladder draining directly into the segment IV inferior and V via the liver bed.14–16 Instead, this mode of spread seems to play a minor role in the early phase of hepatic invasion; lymphatic spread, even in the absence of direct hepatic infiltration, seems to be the main method of diffusion through the liver.17 The rate of liver metastases is about 10% in the absence of hepatic infiltration and increases to 20% in presence of hepatic infiltration.12 According to these findings, wedge resection of the gallbladder bed or resection of segment IVb–V has been advocated by some authors for patients with T1b and T2 tumours.16,18,19 (3) Lymph node metastases are a common finding in gallbladder carcinoma. Lymphatic drainage from the gallbladder is along three main routes.20,21 The right route is the main pathway: the lymph runs in the right half of the hepatoduodenal ligament, along the nodes of the first station (cystic, pericholedochal and proper hepatic artery nodes), into the superior retropancreaticoduodenal or retroportal nodes (second station) and, finally, into the paraaortic nodes (third station). The left route is a less important way of lymphatic drainage: the lymph from the gallbladder, after having reached the nodes of the first and second stations, flows toward lymph nodes medial to the hepatoduodenal ligament (anterior common hepatic artery, posterior common hepatic artery, celiac trunk and superior mesenteric artery nodes). Both right and left routes meet at the lymph nodes around the pancreatic head. The third route is the hilar route: the lymphatic drainage is toward the hepatic hilus. The overall rate of lymph node metastases ranges from 54% to 64%.19,21,22 The extent of the lymph node involvement is strongly correlated with the depth of invasion of the gallbladder carcinoma: a multicentre Japanese study has shown that the rate of lymph node metastases is only 2.5% in pT1a tumours, but it increases to 15.6% in pT1b tumours, to 44.3% in pT2 tumours and to 72% in pT3–4 tumours.18 The pericholedochal lymph node is the most common metastatic node followed by the cystic, posterosuperior pancreaticoduodenal, retroportal, common hepatic artery and celiac trunk nodes (Table 2). Moreover, pT3–4 tumours have a higher N2:N1 ratio than pT2 tumours.5,22 (4) Intraductal spread of gallbladder carcinoma is 440 A. Muratore et al. Table 2. Rate of lymph node involvement in patients with gallbladder carcinoma21,22 Site Group Cystic lymph node Pericholedochal lymph node Proper hepatic artery lymph node Retroportal lymph node (cephalad) Retroportal lymph node (caudad) Posterosuperior pancreaticoduodenal lymph node Common hepatic artery lymph node Celiac trunk lymph node Posteroinferior pancreaticoduodenal lymph node Superior mesenteric artery lymph node Interaorticocaval lymph node Frequency N1 N1 N1 N1 N2 N2 25% 32% 10% 4% 20% 20–35% N2 N2 N2 20% 15% 3% N2 M1 10% 19–25% characteristic of the papillary tumours: the tendency for this histological subtype to grow intraluminally seems to explain the more favourable prognosis.22 Treatment The depth of tumour invasion and the presence of metastatic lymph nodes are the main prognostic factors; however, only the T factor can be easily recognized by pre-operative imaging techniques or intraoperative ultrasonography, and it becomes a useful variable for choosing the most appropriate surgical treatment. Whatever the extension of the resection, the achievement of a negative margin is mandatory for long-term survival: the rate of histological curative resection (R0) is 100% for stage I–II tumours and 48–52% for stage III–IV tumours.5,23 Tis-1 tumours Tis and T1 tumours are usually pathological findings after laparoscopic cholecystectomy for cholethiasis; Tis and T1 lesions are found respectively in 4% and 15–40% of patients resected for gallbladder cancer.5,18,24,25 For pTis and pT1a tumours, cholecystectomy alone offers the greatest probability of cure with low morbidity and mortality rates: the 5-year survivals range from 83% to 100%.16,26–28 Simple cholecystectomy, as adequate treatment for pT1b patients, is still a matter of discussion; Shirai has reported a favourable outcome regardless of the operation done, cholecystectomy alone or extended cholecystectomy, in a group of 11 patients with the tumour invading the muscle layer.26 The results reported by other authors after simple cholecystectomy for pT1b cancer are not as good, with a 5-year survival ranging from 40% to 50%;16,28 Benoist has recently reported the findings of a multicentre retrospective study: of 23 patients with pT1b tumours who underwent cholecystectomy alone, six died of recurrence, whereas, of 13 with pT1a cancers, only one died of recurrence.25 Moreover, another multicentre study has reported a 5-year survival of 18% for 20 pT1 patients treated by cholecystectomy alone.27 The 5-year survival for the subset of pT1b patients increases to 72–100% after a more extended procedure (cholecystectomy with hepatic resection and lymph node dissection).5,15,18,21,28 These results are supported by the findings of a recent multicentre Japanese study of 1686 patients resected for gallbladder cancer: the rate of lymph node metastases was 2.5% in 201 patients with a pT1a tumour but increased to 15.5% in 165 patients with pT1b tumours.18 In conclusion, most of the Tis and T1 tumours are an incidental discovery after laparoscopic cholecystectomy for gallstone disease: simple cholecystectomy is an adequate treatment for patients with pTis or pT1a tumours and no further reoperation is necessary; in the presence of infiltration of the muscle layer (pT1b), cholecystectomy with hepatic resection (wedge resection of the gallbladder bed or segment IVb–V resection) and lymph node dissection (NI station) seems to be associated with a better long-term survival. T2 tumours The rate of metastatic lymph nodes ranges from 40% to 60%, with a 20% rate of metastases to N2 lymph nodes.5,18,22,21 Moreover, some studies have reported that, even in the absence of hepatic infiltration, liver metastases (mainly in the gallbladder bed) are possible.12,17 The 5-year survival for patients with pT2 tumours is 22–40% after simple cholecystectomy but increases to 60–80% after a more extended resection.6,24,25,29,30 Indeed, cholecystectomy with hepatic resection and dissection of N1–2 lymph nodes is the standard treatment for patients with pT2 gallbladder cancer; whether a wedge resection or a segment IVb–V resection should be performed is unclear: no significant benefits in terms of long-term survival have been demonstrated by the use of either of these two procedures.12 Patients with a pT2 tumour discovered after a laparoscopic cholecystectomy should undergo a radical reresection: a recent study has shown the presence of metastatic lymph nodes in 50% of reresected T2 patients.31 T3–4 tumours The treatment of advanced gallbladder carcinomas is a challenging problem, with a 5-year survival rate ranging from 15% to 50% for pT3 tumours and from 7% to 25% for pT4 tumours.18,19 The extent of hepatic resection is still unclear: resection of segment IVb–V should be performed for patients with liver-bed carcinoma; an extended right hepatectomy seems the appropriate treatment to control direct liver infiltration for hilum type carcinomas or liverbed carcinomas with an infiltrating pattern.11,12 The main goal of hepatic resection is the achievement of a negative margin which is the only chance of long-term survival.5,19,32 Since a high rate of lymph node involvement is common for T3–4 carcinomas, dissection of N1 and N2 lymph nodes is mandatory. Lymph node status is an important prognostic factor and, despite the negative results of a multiinstitutional French study, long-term survival is possible;16,19,25 Tsukada has reported that, of 60 patients with lymph node metastases, 11 with N1 spread and four with N2 survived for more than 5-years.5 Indeed, metastases to 441 Radical surgery for gallbladder cancer N1 or N2 lymph nodes discovered by frozen section are not a contraindication to a curative resection. Some Japanese authors have attempted pancreaticoduodenectomy (PD) combined with hepatic resection for patients with metastatic peripancreatic lymph nodes (N2) or direct invasion of adjacent organs (T3–4), in an effort to improve the prognosis.18,33 In a recent study, Shirai reported the results of 17 patients who had undergone combined PD and hepatectomy for stage III or IV carcinoma: of the five 5-year survivors, four had stage IVB disease (N2 lymph nodes).32 However, this approach is associated with a rate of post-operative complications ranging from 34% to 70%.5,33,34 Many Japanese surgeons routinely performed resection of the common bile duct in the course of a curative resection for advanced gallbladder cancer because the lymphatics surrounding the duct are a main route of tumour spread;5,21,35 however, no improvement in long-term survival has been reported as the result of this resection.36 Our and other authors’ approach is the resection of the common bile duct when, on gross examination, the tumour is close to or infiltrates the bile duct (hilum type tumour).37 However, bile duct involvement is associated with a higher rate of positive margins, of metastases to para-aortic lymph nodes and with a worse prognosis.11,20,23,32 The rate of para-aortic lymph node metastases ranges from 19% to 25% in patients with locally advanced disease but the value of para-aortic lymphadenectomy has not been defined yet.21,22,37 All the authors but Yokoyama have reported no long-term survivors among the patients with metastases in this region;21,22,38 the latter author has examined the incidence of lymph node micrometastases: of four patients with positive para-aortic nodes, one was alive and disease-free at 72 months. Therefore, lymphadenectomy of this region may be reasonable for pT3–4 tumours and the discovery by frozen section of a positive node does not preclude a curative resection although the prognosis is poor with very few long-term survivors. Another controversial issue is the use of adjuvant chemotherapy and radiotherapy after curative resection of advanced gallbladder carcinomas: a recent report, at the 1999 ASCO Meeting, on 112 patients who had undergone curative resection and were randomized to receive surgery alone or surgery and chemotherapy has shown a significantly better 5-year survival rate for patients receiving adjuvant chemotherapy.39 However other studies are needed to confirm these results. The aim of adjuvant radiotherapy is to reduce the high rate of loco–regional failure after curative resection: some authors have reported an improved survival after adjuvant radiotherapy but each report was based on a retrospective study with few cases.40–42 A recent study has shown that adjuvant radiotherapy yielded a significantly better 5-year survival rate than resection alone (17.2% vs 0%; P=0.0028) when radiation was used on patients with microscopic cancer residues; however, no significant improvement of survival time was detected using adjuvant radiotherapy in patients with no cancer residues or with macroscopic residues.43 Moreover, the rate of distant metastases was not statistically different between the resection alone group and the adjuvant group. Therefore, Gallbladder cancer Post-operative diagnosis Tis-1a T1b T2 T3-4 Pre- or intraoperative diagnosis pTis-1a* pTis-1b* pT2-3-4 No further therapy Chole Chole + WR + N1 Type of reresection depending on pT Chole + WR or IVb-V + N1-N2 Chole + IVb-V or EH + NI-N2 ± VB or ± PD Fig. 2. Algorithm for management of gallbladder cancer based on depth of the tumour. Chole: cholecystectomy; WR: wedge resection; IVb-V: resection of segment IVb-V; EH: extended right hepatectomy; PD: pancreaticoduodenectomy; VB: resection of common bile duct; N1: dissection of N1 lymph nodes; N2: dissection of N2 lymph nodes; h>: optional treatment; ∗ usually pathological findings, rare pre-operative diagnosis. other studies are needed to evaluate the role of combined radiotherapy and chemotherapy after curative resection. In conclusion, patients with T3–4 tumours should undergo resection of segment IVb–V (liver-bed type) or extended right hepatectomy (hilum type or liver-bed type with deep infiltration) associated to N1–2 dissection. In the presence of a tumour close to or infiltrating the duct, resection of the bile duct is mandatory. Combined PD and hepatectomy can be an efficacious treatment for selected patients with stage III–IV tumours provided that a curative resection can be performed with low morbidity and mortality rates. Recent reports seem to suggest a role for radiotherapy and chemotherapy after curative resection of advanced gallbladder cancer. Laparoscopy and port site recurrence The incidence of port site recurrence of carcinoma after laparoscopic procedure ranges from 8% to 19%;31,44,45 most, but not all, such recurrences are at the site of gallbladder removal.46,47 Many experimental studies have demonstrated that cellular dissemination during laparoscopic cholecystectomy is frequent and that pneumoperitoneum significantly increases tumour implantation and growth at trocar sites.48,49 Moreover, another recent experiment study has reported that peritoneal injuries, i.e. caused by trocar insertion, enhance peritoneal implantation of carcinoma cells.50 Thus, if gallbladder cancer is diagnosed or suspected pre-operatively, an open laparotomy is indicated; if it is diagnosed during laparoscopy, the laparoscopic procedure should be converted to an open procedure with excision of port sites. Patients with a pT1b–4 tumour discovered on the final pathology report need a reoperation with excision of trocar port sites.31,46 442 A. Muratore et al. Conclusions The most appropriate surgical treatment of gallbladder cancer is determined on the basis of the depth of infiltration (Fig. 2). Most Tis or T1 tumours are discovered incidentally after cholecystectomy: no further therapy is requested for pTis or pT1a tumours whereas relaparotomy with wedge resection and N1 dissection is necessary for pT1b. Cholecystectomy with hepatic resection (wedge resection or resection segment IVb–V) and N1–2 lymph node dissection improves the long-term survival of patients with T2 tumours. The only chance of long-term survival for patients with T3 or T4 gallbladder cancer is an extended operation combining an hepatic resection (segment IVb–V or extended right hepatectomy) with a N1–2 dissection, with or without resection of the common bile duct. Pancreaticoduodenectomy combined with hepatic resection seems to improve the survival in patients with metastatic peripancreatic nodes or direct infiltration of adjacent organs, provided that a curative resection can be obtained with low morbidity and mortality rates. Moreover, recent reports seem to suggest a role for radiotherapy and chemotherapy after curative resection of advanced gallbladder cancer. References 1. Destoll M. Rationis mendenchi. In: Batavorum L. (Ed.) Nosocomio practico vendobonensi—Part 1. Honkoop, Hoak et Socios et A et J, 1788. 2. Sheinfeld W. Cholecystectomy and partial hepatectomy for carcinoma of the gallbladder with local liver extension. Surgery 1947; 22: 48–58. 3. Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989–1995. Cancer 1998; 83: 2618–28. 4. Donohue JH, Nagorney DM, Grant SC, Tsushima K, Ilstrup DM, Adson MA. Carcinoma of the gallbladder: does radical resection improve outcome. Arch Surg 1990; 125: 237–41. 5. Tsukada K, Hatakeyama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery 1996; 120: 816–22. 6. Bartlett DL, Fong Y, Fortner JG, Brennan M, Blumgart LH. Long-term results after resection for gallbladder cancer: implications for staging and management. Ann Surg 1996; 5: 639–46. 7. Gagner M, Rossi RL. Radical operations for carcinoma of the gallbladder: present status in North America. World J Surg 1991; 15: 344–7. 8. Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment and prognosis. Cancer 1976; 37: 141–8. 9. UICC. TNM Classification of Malignant Tumors (5th Edn). New York: Wiley & Sons, Inc., 1997. 10. Sons HU, Borchard F, Joel BS. Carcinoma of the gallbladder: autopsy findings in 287 cases and review of the literature. J Surg Oncol 1985; 28: 199–206. 11. Ogura Y, Tabata M, Kawarada Y, Mizumoto R. Effect of hepatic invasion on the choice of hepatic resection for advanced carcinoma of the gallbladder: histologic analysis of 32 surgical cases. World J Surg 1998; 22: 262–7. 12. Yoshikawa T, Araida T, Azuma T, Takasaki K. Bisubsegmental liver resection for gallbladder cancer. Hepato-Gastroenterology 1998; 45: 14–9. 13. Nagai H, Kuroda A, Morioka Y, Shimada H, Ezaki I. Modes of spread of gallbladder carcinoma—a study of autopsied cases. Tan to Sui (J Bil Pancr) 1983; 4: 1227–41. 14. Satoh T. Study of the anatomy of the venous drainage of the gallbladder. J Jpn Bil Assoc 1989; 3: 227–33. 15. Matsumoto Y, Fujii H, Aoyama H, Yamamoto M, Sugahara K, Suda K. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg 1992; 163: 239–45. 16. Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery 1987; 101: 729–37. 17. Shirai Y, Tsukada K, Ohtani T, Watanabe H, Hatakeyama K. Hepatic metastases from carcinoma of the gallbladder. Cancer 1995; 75: 2063–8. 18. Ogura Y, Mizumoto R, Isaji S, Kusuda T, Matsuda S, Tabata M. Radical operations for carcinoma of the gallbladder. World J Surg 1991; 15: 337–43. 19. Chjiiwa K, Tanaka M. Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery 1994; 115: 751–6. 20. Uesaka K, Yasui K, Morimoto T, et al. Visualization of routes of lymphatic drainage of the gallbladder with a carbon particle suspension. J Am Coll Surg 1996; 183: 345–50. 21. Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer 1997; 79: 892–9. 22. Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer 1997; 80: 661–7. 23. Miyazaki M, Itoh H, Ambiru S, Shimuzu H, Togawa A, Gohchi E, Nakajima N, Suwa T. Radical surgery for advanced gallbladder carcinoma. Br J Surg 1996; 83: 478–81. 24. Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder; results of the French Surgical Association Survey. Ann Surg 1994; 219: 275–80. 25. Benoist S, Panis Y, Fagniez PL, the French University Association for Surgical Research. Long-term results after curative resection for carcinoma of the gallbladder. Am J Surg 1998; 175: 118–22. 26. Shirai Y, Yoshida K, Tsukada K, Muto T, Watanabe H. Early carcinoma of the gallbladder. Eur J Surg 1992; 158: 545–8. 27. Cubertafond P, Mathonnet M, Gainant A, Launois B. Radical surgery for gallbladder cancer. Results of the French Surgical Association Survey. Hepato-Gastroenterology 1999; 46: 1567–71. 28. Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Fado K. Diagnostic capability and rational resectional surgery for early gallbladder cancer. Hepato-Gastroenterology 1999; 46: 1557–60. 29. Yamaguchi K, Chijiiwa K, Saiki K, Nishihara K, Takashima M, Kawakami K, Tanaka M. Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg 1997; 84: 200–4. 30. Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of gallbladder. An appraisal of radical second operation after simple cholecystectomy. Ann Surg 1992; 215: 326–31. 31. Fong Y, Hefferman N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy. Aggressive resection is beneficial. Cancer 1998; 83: 423–7. 32. Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Combined pancreaticoduodenectomy and hepatectomy for patients with locally advanced gallbladder carcinoma. Long term results. Cancer 1997; 80: 1904–9. 33. Nakamura S, Nishiyama R, Yokoi Y, et al. Hepatopancreatoduodenectomy for advanced gallbladder carcinoma. Arch Surg 1994; 129: 625–9. 34. Tsukada K, Yoshida K, Aono T, Shirai Y, Uchida K, Muto T. Major hepatectomy and pancreaticoduodenectomy for advanced carcinoma of the biliary tract. Br J Surg 1994; 81: 108–10. 35. Nakamura S, Sagakuchi S, Susuki S, Muro H. Aggressive surgery for carcinoma of gallbladder. Surgery 1989; 106: 467–73. 36. Chijiiva K, Tanaka M. Indications for and limitations of extended cholecystectomy in the treatment of carcinoma of the gallbladder. Eur J Surg 1996; 162: 211–6. 37. Onoyama H, Yamamoto M, Tseng A, Ajiki T, Saitoh Y. Radical surgery for gallbladder cancer 38. 39. 40. 41. 42. 43. 44. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg 1995; 19: 758–63. Yokoyama N, Shirai Y, Hatakeyama K. Immunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibody. Cancer 1999; 85: 1465–9. Amano H, Takada T, Matsushiro T. Nimura Y, Nagakawa T, Nakayama T, Gomi K. Five-year results of a randomized study of postoperative adjuvant chemotherapy for resected pancreatic-biliary carcinomas. ASCO Meeting 1999. Todoroki T, Iwasaki Y, Orii K, et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinomsa. World J Surg 1991; 15: 357–66. Busse PM, Cady B, Bothe A Jr, et al. Intraoperative radiation therapy for carcinoma of the gallbladder. World J Surg 1991; 15: 352–6. Mahe M, Stampfi C, Romestaing P, et al. Primary carcinoma of the gallbladder: potential for external radiation therapy. Radiother Oncol 1994; 33: 204–8. Todoroki T, Kawamato T, Otsuka M, Koike N, Yoshida S, Takada Y, et al. Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. HepatoGastroenterology 1999; 46: 1585–91. Lundberg O, Kristofferson A. Port site metastases from 45. 46. 47. 48. 49. 50. 443 gallbladder cancer after laparoscopic cholecystectomy. Results of a Swedish survey and review of published reports. Eur J Surg 1999; 165: 215–22. Yamaguchi K, Chijiiwa K, Ichimiya H, et al. Gallbladder carcinoma in the era of laparoscopic cholecystectomy. Arch Surg 1996; 131: 981–4. Z’graggen K, Birrer S, Maurer CA, Wehrli H, Klaiber C, Baer HU. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery 1998; 124: 831–8. Jacobi CA, Keller H, Monig S, Said S. Implantation metastasis of unsuspected gallbladder carcinoma after laparoscopy. Surg Endosc 1995; 9: 351–2. Doudle M, King G, Thomas WM, Hewett P. The movement of mucosal cells of the gallbladder within the peritoneal cavity during laparoscopic cholecystectomy. Surg Endosc 1996; 10: 1092–4. Bouvy ND, Marquet RL, Jeekel J, Bonier HJ. Laparoscopic surgery is associated with less tumor growth stimulation than conventional surgery: an experimental study. Br J Surg 1997; 84: 358–61. Aoki Y, Shimura H, Li H, Mizumoto K, Date K, Tanaka M. A model of post-site metastases of gallbladder cancer: The influence of peritoneal injury and its repair on abdominal wall metastases. Surgery 1999; 125: 553–9. World Wide Web Site Reviews In order to help and to encourage our readers to make the best use of the cancer related and surgical oncology resources on the Internet, we are pleased to offer to publish short Web Site Reviews in the Highwayman series. A review may address any site in the broad subject range of our remit or in relation to our recently published articles. The site under review might be a search engine; an information or reference source, of historical or biogeographical interest; a cancer charity; a grant giving body; a complementary or alternative medicine source; or offbeat. Kim et al. have recently published a series of criteria for evaluating health related web sites, which provide a useful framework for would be reviewers. They include: —the content of the site, including quality, reliability, accuracy, scope and depth; —design and aesthetics, layout, interactivity, presentation, appeal, graphics and use of media; —disclosure of authors, sponsors and developers; —currency of information and maintenance of the site; —the authority of the source; —the ease of use, including facility to download, navigability and functionality; —quality of links to other sites; —attribution and documentation; —appropriateness for intended audience; —contact addresses and feedback mechanisms; —user support; —unique features. Reviews should be between 200 and 500 words, and without illustrations or screen snapshots. They may be submitted for publication either as signed reviews or anonymously under the ‘Highwayman’ imprint. They offer a particular opportunity for your trainees and younger web wizards to exercise their pens and keyboards in creative writing. Reference 1. Kim P, Eng TR, Deering MJ, Maxfield A. Published criteria for evaluating health related Web sites. BMJ 1999; 318: 647–9.