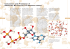IB Topic 4: Bonding
Transcription
IB Topic 4: Bonding
IB Topic 4: Bonding pure carbon is covalently bonded in three of different forms (allotropes) 1. graphite 2. diamond 3. fullerenes To buy lots of super duper expensive carbon allotropes, CLICK HERE possesses a layer structure the layers of carbon atoms are arranged in an repeating fashion more compact structure and dense than graphite one of the hardest materials known highly stable chemically most famous is the “buckeyball” › discovers awarded Nobel Prize in 1996 › over 1000 fullerene compounds have been made composed of carbon atoms that form a hollow, cage-like structure › interesting feature of fullerenes is their ability to enclose other atoms forces that occur between molecules much weaker than intramolecular (within the molecule) forces › it takes 464 kJ/mol to break the H-O bonds within a water molecule and only 19 kJ/mol to break the bonds between water molecules the strength of the intermolecular forces determines the physical properties of the substance › melting, boiling, reacting, solubility, conductivity, volatility this will be covered in next PowerPoint van der Walls YouTube (:20) also also known as London Dispersion Forces even nonpolar molecules have forces that hold them together the distribution of electrons around an individual atom, at a given instant in time, may not be perfectly symmetrical › this can produce a temporary, instantaneous dipoles (polar molecule) › this can then induce a nearby molecule to be polar and therefore a very weak attraction between the two molecules and so on, and so on… Sticky secret. Tiny hairs on geckos' feet help maximize contact with surfaces, allowing van der Waals forces to go to work. attractive forces between the positive end of one polar molecule and the negative end of another polar molecule must be in close proximity for the dipoledipole forces to be significant stronger than van der Waal's forces YouTube Hydrogen Bonding (1:40) YouTube Hydrogen Bonding Video (:58) a specific type of dipole-dipole type interactions stronger than other dipole-dipole and/or dispersion forces the hydrogen in a molecule (e.g. H-F, H-O or H-N) is bonded to a small, highly electronegative element (usually an F, O or N atom) on another molecule