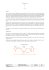Significant Reduction of Symptoms of Scarring with
Transcription
Significant Reduction of Symptoms of Scarring with
case series IC A TE Significant Reduction of Symptoms of Scarring with Electrical Stimulation: Evaluated with Subjective and Objective Assessment Tools in a Prospective Noncontrolled Case Series N O T D From the 1Manchester Institute of Biotechnology, University of Manchester, Manchester, UK; 2 University Hospital of South Manchester NHS Foundation Trust, Institute of Inflammation and Repair, Manchester Academic Health Science Centre, University of Manchester, Manchester, UK; 3 Fenzian Ltd, Hungerford, Berkshire, UK; 4Medical Statistics, University Hospital South Manchester, Manchester, UK Abstract: Introduction. Fenzian wave (FW) electrical stimulation has been shown to influence cutaneous wound healing. The authors previously published a case series investigating the effect of FW on symptomatic abnormal skin scars (raised dermal scars [RDS]) using spectrophotometric intracutaneous analysis (SIAscopy). In addition, a human volunteer sequential biopsy study in acute cutaneous wounds was conducted, which demonstrated that FW increased vascularity. The aim of this study was to evaluate the effectiveness of FW on symptomatic RDS using full-field laser perfusion imaging (FLPI) to assess changes in dermal blood flow. Methods. Eighteen patients with RDS and long-term pain and pruritus participated. Time points analyzed were day 0, weeks 1 and 2, and months 1 and 2. Symptoms were monitored using a subjective numerical rating scale. Additionally, a Manchester Scar Scale and digital photography were used. Objective noninvasive measures captured quantitative data: SIAscopy to measure melanin, hemoglobin and collagen levels, and FLPI to assess the dermal blood flow. Results. There were statistically significant reductions in pain scores (from day 0 to month 1, P = 0.007) and pruritus scores (from day 0 to week 1, P = 0.007; and day 0 to month 1, P = 0.002). The trend of melanin levels demonstrated an increase from day 0 to week 1, hemoglobin levels showed an increase from day 0 to week 2, and hemoglobin flux increased from day 0 to week 2 (not statistically significant). Conclusion. This report demonstrates that FW electrical stimulation significantly reduces the symptoms of pain and pruritus in patients with RDS. This unique treatment has the potential for management of symptomatic skin scarring. U WOUNDS 2013;25(8):212-224 PL Sara Ud-Din, MSc1,2; Pamela Giddings Dip, HE3; James Colthurst, FRCS3; Sigrid Whiteside, MSc2,4; Julie Morris, MSc2,4; Ardeshir Bayat, PhD, MBBS, MRCS1,2 D O Address correspondence to: Ardeshir Bayat, PhD, MBBS, MRCS Plastic and Reconstructive Surgery Research Manchester Institute of Biotechnology University of Manchester 131 Princess Street Manchester M1 7ND ardeshir.bayat@manchester.ac.uk Disclosure: Funding for this study was provided in part by Fenzian Ltd. Key words: electrical simulation, Fenzian wave, abnormal skin scarring, raised dermal scarring, pain, pruritus, noninvasive devices 212 WOUNDS® www.woundsresearch.com Bayat et al A TE digital photography, numerical rating scales, scar scales, and spectrophotometric intracutaneous analysis, which is a noninvasive device.27 The results showed this intervention reduced symptoms and scar scores significantly, and indicated that these positive effects warranted further investigation. Furthermore, the authors have conducted a human volunteer controlled trial, using a temporal punch biopsy model to evaluate the effects of FW on acute cutaneous wound healing. It was demonstrated through the use of full-field laser perfusion imaging (FLPI) that vascularity is increased with FW. The histological results also showed that FW accelerated healing by increasing angiogenesis and reducing inflammation.28 Therefore, it was postulated that it would be beneficial to investigate if vascularity is affected in patients undergoing FW treatment for their symptomatic RDS using FLPI, as this had not been evaluated in the previous case series. The purpose of this study was to explore these effects in an additional cohort of patients. PL IC E ach year in the developed world, up to 100 million people develop skin scarring as a result of burns, self-harm, and other injuries or trauma.1-3 Skin scarring presents a clinically challenging problem. Patients with abnormal skin scars, particularly raised dermal scars (RDS), which include hypertrophic and keloid scars, often report considerable discomfort due to the physical morbidity of pain, inflammation, and pruritus.4 These scars can also impact a patients’ quality of life and their psychosocial symptoms including anxiety and low self esteem.5-9 Many therapies are utilized in the management of RDS, including steroid injections, pressure therapy, and surgical interventions.10 However, these treatments can be unsatisfactory, and if used alone, especially in the case of keloid scars, can lead to recurrence. Recurrence rates following surgical excision and steroid injections have been reported to be as high as 50%.10,11 Thus, there is a need for safe, alternative therapies that have a low side effect profile with reduced risk of recurrence. Electrical simulation (ES) is one therapy that has been used successfully in the past for treatment of RDS.12-18 Different types of ES have been shown to have beneficial effects on wound healing.12-24 Direct current (DC), which is a unidirectional flow of charge toward the positive pole, has been useful in treating chronic skin ulcers.19 Alternating current (AC), where the charge flow constantly changes direction reversing the polarity, has been successfully used in managing diabetic and neuropathic foot ulcers.20,21 Additionally, pulsed current, where the flow of charged particles intermittently stops for a limited period of time, has been beneficial in enhancing the healing of chronic wounds.22-24 A novel ES waveform that degenerates with time, called the Fenzian wave (FW), has already been used in the treatment of wound healing.25-28 A previous case review demonstrated the use of FW in improving a range of symptoms, showing promise in the treatment of cutaneous injuries.25 A recent in vitro study also identified that FW had beneficial effects on skin and keloid fibroblasts.26 Both types of fibroblasts were electrically stimulated with alternating currents, as well as direct current and FW. Fenzian wave was shown to decrease collagen levels; therefore, it is considered to be a potentially useful method for reducing the rate of collagen formation in keloid scars. The authors recently published a case series that investigated the effect of FW ES on symptomatic abnormal skin scars.27 This was evaluated through the use of Methods D O N O T D U Patients were recruited from October 2010 to June 2011 through the specialist scar service at the University Hospital of South Manchester NHS Foundation Trust, in Manchester, England, UK. The patients were provided with written and verbal information about the FW treatment and were given the opportunity to ask questions prior to treatment, during which time it was also established if they fulfilled the inclusion and exclusion criteria. Patients of any age, sex, and medical history were eligible. The inclusion criteria were: any patient who had symptomatic scars that were RDS only, including keloid or hypertrophic scars and RDS of different causations, such as acne, surgery, and trauma; patients who had scars that had not previously responded to treatments for their symptoms, (eg, steroid injections or surgery); and those who requested further noninvasive treatment. The exclusion criteria were: patients who were taking medications such as antibiotics or steroids as they are known to reduce the electrical activity in the skin29; patients who were pregnant or planning to conceive; patients with implanted electrical devices (ie, pacemaker, cochlear implant); and patients with other types of scars. Fenzian Wave Electrical Stimulation Device The Fenzian treatment system (Fenzian Ltd, Hungerford, UK) is Conformité Européenne (CE) approved and 510(k) registered with the United States Food and Vol. 25, No. 8 August 2013 213 Bayat et al Intervention N O T D U PL IC A TE The treatment was performed by a single practitioner who was trained using a protocol tailored to each patient.The treatment process is outlined in a flowchart (Figure 1). The patients were treated while sitting in a chair or lying down, whichever was most appropriate to view the location of the scar. The device, due to this biofeedback electrical mechanism, guides the length of treatment. Furthermore, the device is set at a strength that is comfortable for the patient. All patients gave written consent for photographs and noninvasive imaging to be carried out throughout their treatment. Patients did not undergo any other scar therapies for the duration of their ES treatment. Patients were offered 2 treatments a week for the first month and this was reduced over time according to their symptom reduction.Additionally, they attended a follow-up appointment with the senior clinician at 4 months. O Figure 1. A flowchart depicting the Fenzian wave electrical treatment process. D Drug Administration. The University Hospital of South Manchester Trust Medical Engineering also approved the system for use. This device is a low intensity transcutaneous electrical stimulation device that detects changes in skin impedance using a microcurrent generator. The electrode is 45 mm x 22 mm in size, and impulses are applied of approximate duration of 1/600th of a second and of 20-80V amplitude. 214 WOUNDS® www.woundsresearch.com Assessment Tools Manchester Scar Scale. Digital photographs were obtained to monitor the appearance of the scar. The scars were also clinically evaluated at each appointment using the Manchester Scar Scale,30 which assesses the color, contour, distortion, texture, and whether the scar has a matte or shiny appearance. Each of these criteria score from 1 to 4 and a combined total score ranges from 5 to 18, with 18 being the most severe. Numerical Rating Scale. The patient’s subjective rating of their pain and pruritus stimulation were obtained at each visit using the Numerical Rating Scale (NRS).A score of 0 indicates no pain or pruritus and a score of 10 indicates the worst pain or pruritus. It was also recorded whether these symptoms were constant or intermittent. Noninvasive imaging techniques were also used at various time points throughout the patients treatment on the same scar site each time, using spectrophotometric intracutaneous analysis and FLPI. Spectrophotometric intracutaneous analysis. Spectrophotometric intracutaneous analysis (SIAscopy) Bayat et al (Astron Clinica Ltd, Cambridge, UK) is a noninvasive scanning technique that analyzes light reflected from the skin.31 Numerical values for the pigmentary status—hemoglobin, melanin, and collagen—in the first 2 mm of the skin32 are provided for quantitative analysis. The software provides high-resolution maps of various components of the skin, such as the concentration and location of melanin in the papillary dermis and epidermis, also the concentration of blood and collagen in the papillary dermis.31 The light-based technology probe, which is 11 mm in diameter, was placed on the same scar site for each patient at each visit. Full-field laser perfusion imaging. The measure of blood flow in the cutaneous scar tissue is linked to the vascularization present. Full-field laser perfusion imaging (Speckle Contrast Imager-1,Moor Instruments Ltd,Axminster, UK) was used to obtain quantitative values for the blood flow present in the scar at each visit. The FLPI was positioned perpendicular at a set distance from the scar site and the focus was adjusted to incorporate the specific scar area. Images were produced of the blood flow in the microvessels in the surface layer of the tissue. Ten consecutive images were obtained over a period of 10 seconds, and the average blood flow was calculated when analyzing the images using the software (version 3.0).The perfusion unit is expressed as ‘flux.’ Table 1. Summary of patient demographics. Statistical Analysis Table 2. Abnormal scar data information. No. of patients Total Gender Male Female White Other Fitzpatrick Skin Type I II III IV V VI Percent (%) 18 100.0 8 10 44.4 55.6 12 6 66.7 33.3 0 12 1 3 2 0 0 66.7 5.6 16.7 11.1 0 12 5 72.2 27.8 10 4 3 1 55.6 22.2 16.7 5.6 3 5 4 6 16.7 27.8 22.2 33.3 IC A Ethnicity Frequency TE Demographics I-III IV-VI No. of scars 1 2 3 Multiple Age group (years) ≤ 25 26-35 36-45 ≥ 46 D U PL Fitzpatrick Skin Type group D O N O T Trends were documented between demographic characteristics using descriptive statistics. One scar per subject was selected to be included in the statistical analysis. Each patient’s most problematic scar, with the worst symptoms, was chosen to be analyzed. The changes from day 0 to each time point were analyzed separately using nonparametric summary statistics and pairwise Wilcoxon signed-rank tests to assess differences in pain, pruritus, scar score, blood flow, melanin, hemoglobin, and collagen levels. All analyses were performed using the statistical package SPSS version 15 (SPSS Inc, Chicago, IL) and a 1% significance level was used for the P-values to adjust for multiple comparisons. It was only feasible for the same person to conduct all the treatments and assessments, and this possibly could lead to bias. To minimize bias, independent statisticians carried out all of the statistical analysis. Results Demographics. Demographic data is presented in Table 1. Nineteen patients were treated with FW. Combined, Details No. Location Sternum Shoulder Abdomen Breast Other 10 2 1 2 3 Cause Trauma Surgery Acne 6 5 7 Age of scar < 11 months 1-3 years > 3 years 2 5 11 Type of scar Keloid Hypertrophic 14 4 Symptoms Redness only Pain and itch Pain only Itch only 2 15 0 1 Previous scar excision Yes No 7 11 Vol. 25, No. 8 August 2013 215 Bayat et al Table 3. Number of Fenzian Wave electrical stimulation treatments patients received over the 2-month period Week 1 N = 10 Week 2 N = 12 Month 1 N = 13 1.0 3.0 3.5 6.0 9.5 Minimum 1 2 2 3 4 Maximum 1 3 5 10 14 Table 4. Abnormal scar data information. Day 0 pain score Day 0 pruritus score Percent (%) 2 7 5 4 11.1 38.9 27.8 22.2 0 1-3 4-6 7-10 Frequency IC Frequency 1 1 3 13 PL Score Group A Median Pain score * Increase Same Decrease Month 1 Increase Same Decrease Month 2 Increase Same Decrease N O Pruritus score * Frequency Percent (%) 1 2 7 10.0 20.0 70.0 0 1 9 0 10.0 90.0 1 4 5 10.0 40.0 50.0 0 4 7 0 36.4 63.6 1 1 10 8.3 8.3 83.3 0 2 11 0 15.4 84.6 1 1 7 11.1 11.1 77.8 1 1 7 11.1 11.1 77.8 U Week 2 5.6 5.6 16.7 72.2 Percent (%) D Increase Same Decrease Percent (%) Frequency T Week 1 Month 2 N = 10 TE Day 0 N = 18 * Changes from day 0, excluding score of 0 on day 0 D O patents had more than 28 symptomatic RDS which were monitored; however, only 1 scar per patient—the most symptomatic scar as identified by the patients during the initial consultation—were included in the statistical analysis. One patient was excluded from the analysis after opting to have surgical excision of their scar instead. This resulted in a case series of 18 patients, with 18 scars included in the statistical analysis. Patients’ mean age was 39 years, and ranged from 22-80 years.The modal Fitzpatrick Skin Type was II (66.7% of patients; fair skin, burns easily, and tans poorly), and the majority of patients were female (n = 10) and white (n = 12). Presenting abnormal scars. Presenting abnormal scars data is outlined in Table 2. The majority of patients presented with their symptomatic RDS being 216 WOUNDS® www.woundsresearch.com keloid (n = 14). The scars were predominantly due to acne (n = 7); however, the cause of the scars also included trauma (n = 5) and surgery (n = 6). The area most affected by the scarring was the sternum (n = 10), with the majority of the scars being long-standing for more than 3 years (n = 11). Prior to commencing ES treatment, 15 patients complained of both pain (defined as constant and persistent pain which had been present for more than 3 months) and pruritus (defined as a constant itch which had persisted for ≥ 3 weeks); 1 patient complained of only pruritus; none of the patients complained of only pain; and 2 patients expressed they had no symptoms except redness.The median baseline pruritus score was 4 (range 0-10) and median baseline pain score was 8 Bayat et al Table 5. Symptomatic outcomes demonstrating pain scores significantly reduced from day 0 to month 1 and pruritus scores showed statistically significant reductions from day 0 to week 1, and to month 1, respectively. Week 1 Week 2 Month 1 Month 2 Median Minimum Maximum N = 18 4.0 0 10 N = 10 3.5 0 10 N = 12 3.0 0 8 N = 13 3.0 0 9 N = 10 3.0 0 10 Median Minimum Maximum P-value N = 16 5.0 1 10 - N = 10 3.5 0 10 0.091 N = 10 3.0 1 8 0.058 N = 12 3.0 0 9 0.007 ^ N=9 3.0 0 10 0.120 Median Minimum Maximum N = 18 8.0 0 10 N = 10 4.0 0 10 N = 12 3.0 0 10 N = 13 5.0 0 9 N = 10 2.5 0 10 Median Minimum Maximum P-value N = 17 8.0 3 10 - N = 11 3.0 2 10 0.016 N = 13 5.0 0 9 0.002 ^ N=9 3.0 0 10 0.030 Median Minimum Maximum P-value N = 18 14.0 6 16 - N = 12 14.5 6 16 0.317 N = 13 14.0 12 16 > 0.999 N = 10 14.0 6 16 > 0.999 Manchester Scar Score total N = 10 4.0 0 10 0.007 ^ N = 10 14.5 12 16 0.317 D Pruritus score, excluding score 0 on day 0 U Pruritus score A IC Pain score, excluding score of 0 on day 0 PL Pain score TE Day 0 T P-values from paired Wilcoxon signed ranks tests vs day 0 ^ Statistical significance at 1% level D O N O (range 0-10). The median baseline score was 14 (range 6-16) using the modified Manchester Scar Scale. Additionally, a median of 10 (range 5-22) treatments were performed on the 18 patients over 2 months (Table 3). As this data was for a case series and not a structured patient trial, patient visits occurred at various time points, rather than at planned time intervals. All patients had a treatment on day 0; however the other analysis time points were chosen to use the most available data at times similar to those in previous publications. The time points chosen were week 1 (7 days ± 1 day following the first treatment), week 2 (14 days ± 1 day following the first treatment), month 1 (28 days ± 2 days following the first treatment) and month 2 (56 days ± 7 days following the first treatment ). For multiple available measurements within a time window, the earliest and closest day to the nominal time was used. The same day was chosen for all parameters. Patients were reviewed at month 6, although only patient data at the chosen time points above were included in the summaries and analyses due to decreased number of patients returning at month 6. Analysis of Symptomatic Outcomes Table 4 shows the patient-reported symptomatic scores and the changes from baseline to each time point. Fifteen patients commenced with pain prior to treatment. Ten of these were included in the week 1 analysis and 7 (70%) reported a decrease in pain at week 1. One patient (10%) experienced an exacerbation of their pain symptoms following initial treatment. Sixteen patients reported pruritus symptoms prior to treatment, and of the 10 recorded, 9 (90%) reported an improvement at week 1. No patients had an increase in pruritus symptoms at this point. At month 1, 10 (83.3%) of the patients who reported pain symptoms initially experienced an improvement. Eleven (84.6%) of the patients who initially reported pruritus symptoms found their symptom decreased. At this time point, the median number of treatments was 6 (range of 3-10). Vol. 25, No. 8 August 2013 217 Bayat et al N O Figure 2. T D U PL IC A TE patients reported no pruritus symptoms; all of these patients had a considerable reduction in pruritis after a median of 9.5 treatments (range of 4-14 treatments). Patients were followed up to month 6 to assess if any changes had been sustained, although this data was not included in the statistical analysis due to a decreased number of patients. All patients who were reviewed stated their symptoms remained reduced or had completely resolved. Pain scores showed statistically significant reductions from day 0 to month 1 at the adjusted 1% significance level (P = 0.007). However, there were no statistically significant changes in pain scores from day 0 to weeks 1 and 2, or month 2, respectively (Table 5). Pruritus scores showed statistically significant reductions from day 0 to week 1 (P = 0.007) and from day 0 to month 1 (P = 0.002), respectively, at the adjusted 1% significance level. However, there were no statistically significant changes in pruritus scores from day 0 to week 2 or from day 0 to month 2, respectively (Table 5). Manchester Scar Scale outcomes.Three patients noted some reduction in scar color by the completion of treatment compared to baseline (Figure 2a), although, there were no significant changes noted in the Manchester Scar Scale throughout the duration of treatment. The scar score total showed no statistically significant changes from day 0 to weeks 1 and 2, or to months 1 and 2, respectively (Table 5). SIAscopy analysis. SIAscopy was used to capture data to identify any changes in patterns from baseline to the other time points (Figure 2). Trends in melanin levels demonstrated an increase from day 0 to week 1 (Figure 3a), hemoglobin levels showed an increase from day 0 to week 2 (Figure 3b), and collagen levels remained approximately the same throughout (Figure 3c). Although, there were no statistically significant changes observed in melanin, hemoglobin, or collagen levels from day 0 to weeks 1 and 2, or to months 1 and 2 (Table 6). Full-field laser perfusion imaging analysis. Full field laser perfusion imaging was used to monitor the blood flow (flux) across the scars and observe any changes from baseline to the other time points (Figure 4). The trend in hemoglobin flux increased from day 0 to week 2 (Figure 3d), although, hemoglobin flux mean levels showed no statistically significant changes. Whilst undergoing the therapy, 12 patients experienced tiredness post-treatment which lasted for ap- a) Images of a 23-year-old male with a facial hypertrophic scar that caused by acne. The images show a reduction in scar color from day 0 to month 2 following completion of Fenzian wave electrical stimulation treatment. O b) Spectrophotometric Intracutaneous Analysis (SIAscopy) images displaying scar color that has reduced from day 0 to month 2. c) SIAscopy images demonstrating a reduction in melanin levels from day 0 to month 2. D d) SIAscopy images showing a reduction in hemoglobin levels over the 2-month period. e) SIAscopy images displaying the collagen levels. At month 2, 7 (77.8%) patients reported no pain, therefore resolution of this symptom was considered achieved at this time point. Also at month 2, 8 (47%) 218 WOUNDS® www.woundsresearch.com Bayat et al N O Figure 3. T D U PL c.d. IC A TE a.b. a) The graph shows the trend of median melanin levels which increase from day 0 to week 1. b) The graph shows the trend of median hemoglobin levels that increases from day 0 to week 2 with a subsequent reduction thereafter. c) The graph shows the trend of median collagen levels that showed no overall changes. O d) The graph shows the trend of median hemoglobin flux levels demonstrating an increasing trend from day 0 to week 2, which was similar to that of hemoglobin levels. D proximately 2 hours, and 5 patients reported a tingling sensation at the treatment site which they stated was not painful and only lasted for approximately 1 hour after treatment. Four patients noted that, following the first treatment, their symptoms subsided over a period of 4 days but began to return approximately 1 week post-treatment, although not as intensely as at baseline. No adverse side effects were reported. Discussion Electrical stimulation in the form of FW showed reduction of pain and pruritus scores from baseline to the completion of treatment in symptomatic skin scars. Pain and pruritus symptom scores were significantly reduced in all patients from baseline to 1 month following the first treatment. These results were based on the patients’ subjective numerical rating Vol. 25, No. 8 August 2013 219 Bayat et al Table 6. Summary of results for changes in melanin, hemoglobin, collagen, and hemoglobin flux (or blood flow). Week 1 Week 2 Month 1 Month 2 Median Minimum Maximum P-value N = 18 268.18 -145.0 842.9 - N=9 309.21 210.6 843.0 0.214 N=5 297.02 144.3 405.4 0.999 N=5 301.39 176.7 349.2 0.893 N=6 291.43 120.2 842.9 0.028 Median Minimum Maximum P-value N = 18 165.96 0.4 510.2 - N=9 150.21 0.0 223.7 0.859 N=5 200.56 107.8 213.1 0.273 N=5 156.80 121.3 213.8 0.686 N=6 163.19 0.0 214.4 0.249 Median Minimum Maximum P-value N = 18 184.93 108.2 987.7 - N=9 197.15 127.57 409.82 0.086 N=5 173.36 122.0 249.1 0.999 N=5 160.44 111.2 172.8 0.138 N=6 184.25 138.2 223.1 0.028 Median Minimum Maximum p-value N = 11 221.85 81.6 949.4 - N=2 475.39 357.8 592.9 - N=3 276.39 89.5 516.4 - N=3 249.46 167.6 327.1 - Collagen N=6 192.46 67.0 250.4 0.600 U Flux A IC Hemoglobin PL Melanin TE Day 0 D P-values from paired Wilcoxon signed ranks tests vs day 0 ^ Statistical significance at 1% level D O N O T scale scores, which are clinically valid; in order to be classed as clinically significant, a reduction of 2 points on the scale was necessary.33 The use of the NRS to assess symptoms has been widely recognized.33-35 Recently, Phan et al24 evaluated the validity and reliability of various subjective scales used for itch assessment and found the NRS to be highly reliable with concurrent validity. Hjermstad et al35 also found the use of the NRS to be applicable in most settings for the assessment of pain intensity. Patients were reviewed 6 months post-treatment to ascertain if their symptoms remained resolved. All patients expressed they did not have recurrence of their symptoms of pain or pruritus. However, it would be beneficial to conduct additional follow-up after 1 year to assess if these changes had been sustained. Forty-five percent (45%) of patients responded to treatment and had resolution of their symptoms within 1 month of commencing treatment. The authors noted the remainder of the cohort’s symptoms were reduced by the 2-month period. This longer time scale may be due to a heterogenous cohort of patients that included varying ages, location of scars, age of scars, and type of scars. Interestingly, the sample group consisted of a 220 WOUNDS® www.woundsresearch.com majority of patients who had long-standing scars with persistent symptoms for many years. These symptoms took longer to resolve than patients with more recent scars. It was also noted that the length of time pain symptoms took to reduce varied according to the age of the patients. Additionally, the more elderly patients’ symptoms required longer to subside than younger patients’ symptoms. Furthermore, the location of the RDS was a contributing factor in how quickly patients responded to treatment. Patients with long-standing sternal scars found their symptoms took a more extended time to diminish compared to patients with scars in less stress-prone anatomical locations such as the breast or abdomen. There were no physical scar changes noted indicating a need for longer follow-up as it is thought that the device may target the problematic symptoms first before the appearance of the scar. The authors used objective, noninvasive devices to quantify the effects of FW on symptomatic abnormal skin scarring.These devices measured the hemoglobin, collagen, and melanin levels, as well as the blood flow present at the scar sites. There were trends in melanin levels demonstrating an increase from day 0 to week 1, and hemoglobin levels and blood flow showed an A IC PL increase from day 0 to week 2. The reduction in pain and pruritus was noted in keloid and hypertrophic scars which often tend to be raised and red, therefore indicating reduced inflammation. The trends indicated an increase in hemoglobin and blood flow up to the 2 following initial treatment, which corroborates this. However, histological analysis would be beneficial to investigate these findings further. While searching the literature, the authors noted a trend in increasing blood flow up to week 2 during FW treatment. Previous research has shown that ES improves blood flow and wound tensile strength.36-38 These studies used FLPI to measure this effect, which the authors chose to do in the current case series, and demonstrated a significant increase in blood flow following treatment. A previous study also found that low frequency ES increases blood flow to a wound and vasodilation occurs through the C fibers.39 The authors found a trend of increasing hemoglobin up to 2 weeks following initial treatment with FW with a subsequent decrease thereafter. The increase in hemoglobin could be due to a proinflammatory response that stimulates the tissue.40,41 It is thought that substance P, a neuropeptide, controls pain through the C fibers42 and can be a factor in increasing the neuroinflammatory response in raised scars due to decreased endopeptidase.43 It has also been assumed that pruritus is signaled by a subpopulation of polymodal nociceptive C fibers.44 The significant reductions in pain and pruritus symptom scores suggest a decrease in inflammation at the 1-month period following the initial treatment with FW. This theory corresponds with the decreasing trend seen in hemoglobin levels and blood flow in the 2-week period after FW treatment. The authors have previously shown that FW accelerates acute cutaneous wound healing evidenced by reduced inflammation, enhanced angiogenesis, and advancing the remodeling phase.28 Furthermore, their findings show a statistically significant increase in blood flow at day 14 which corroborates with the trend seen in this case series. The lack of significance using the noninvasive devices may have been due to a reduced number of data at the later stages of treatment. Some patients were not returning at the predetermined specific time points due to work commitments and travel problems; therefore, a larger scale study would be beneficial in order to account for this. Hemoglobin, provides red coloration to human skin via the vascular network of microcapillaries, and mela- TE Bayat et al D O N O T D U Figure 4. The Full-field laser perfusion imager was used to monitor the blood flow across the scars and observe any changes from baseline to the other time points. This is a 42-year-old female with a keloid scar on the breast. The images generated show more intense color at week 2 indicating higher blood flow with a subsequent reduction to month 2 by the end of treatment. nin provides varying degrees of brown coloration at the surface of the skin.45 An increase in melanin was noted at week 1 after FW treatment commenced, as well as a high hemoglobin level when increased pigmentation was noted early in the treatment process. Interestingly, collagen levels remained approximately the same throughout treatment. The majority of the scars were keloid and hypertrophic, which have higher amounts of collagen.46,47 In a previous in vitro study, the authors showed that FW suppressed excessive collagen I formation in keloid disease. Histological analysis would be beneficial in this study to generate more information. There were no significant changes in objective scar scores using the Manchester Scar Scale. Nevertheless, the previous case report using FW on symptomatic abnormal skin scars showed significant reductions in scar scores in particular, and a reduction in scar color over the 2-month period, with the greatest reductions in hypertrophic and surgical scars.27 In 3 of the patients, the authors noted a reduction in scar redness; however, a longer follow-up and increased number of Vol. 25, No. 8 August 2013 221 Bayat et al Conclusion IC A TE All patients with symptomatic RDS who were treated with FW demonstrated a significant reduction of symptoms of pain and pruritus. Patients with keloid and hypertrophic scars in less stress-prone anatomical locations had the greatest reduction in symptoms and younger patients with new scars experienced earlier symptom resolution. This treatment can be beneficial as it may negate the need for long-term pain medications, and has the potential for management of symptomatic skin scarring. Previous Presentation This paper was previously presented at: Symposium of Advanced Wound Care/Wound Healing Society Meeting; April 19-22, 2012; Atlanta, GA. PL treatments may have been beneficial in order to investigate these findings further. This particular study was based on a case series of all patients with symptomatic raised dermal scarring who were treated with ES. Treatment was provided for consecutive cases and was not preplanned. The common denominator with all of these cases was the presence of a symptomatic raised dermal scar, which appeared to be responding to the application of ES. This study provides preliminary data obtained with objective noninvasive devices and subjective assessment tools. However, in order to expand on these results, a larger controlled trial would be beneficial, including a control group with a placebo device to further investigate the effects of this treatment. There was no data obtained using the objective tools on normal skin over time; however, this could be incorporated into a future study. The use of objective assessment tools in this study reduced the possibility of bias, although it was only feasible for the same person to conduct all the treatments and assessments, and this may have led to bias. To minimize this, all of the statistical analysis was carried out by an independent statistician who is named as a coauthor. A limitation was that only 1 scar per patient could be included in the statistical analysis. Furthermore, it would be pertinent to assess whether response to treatment is affected by how often the individual receives treatment, as well as the age of the scar. In addition, a longer-term follow-up, plus histological evidence pre- and post-therapy, may provide further information as to the utility of FW in the treatment of symptomatic abnormal skin scars. In this case series, the authors have shown significant reduction in symptom scores. The therapeutic effect of this treatment is measured by relying on the patients’ reports and it has been argued that subjective measurements can lead to numerous variables that can make evaluation difficult.48 In addition, it is well known that any procedure may generate placebo effects.49 It has been suggested that a placebo has an analgesic effect; therefore, it is important to determine whether the ES treatment has an effect equal to or greater than that of the placebo.48 However, the effects found in this case series cannot be readily ascribed to placebo effects since they were sustained for a long period of time even when the treatments had ceased. Additionally, the studies the authors have previously conducted both clinically and in vitro have demonstrated statistically significant positive effects following FW.26-28,50 References 1. D O N O T D U 2. Gangemi EN, Gregori D, Berchialla P, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg. 2008;10(2):93-102. Sund B. New Developments in Wound Care. London: PJB Publications; 2000. Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ. 2003;326:88-92 Murray JC. Keloids and hypertrophic scars. Clin Dermatol. 1994;12(1):27-37. Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433-438. Rhee P, Brown C, Martin M, et al. QuickClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64(4):1093-1099. Brown BC, Moss TP, McGrouther DA, Bayat A. Skin scar preconceptions must be challenged: importance of self-perception in skin scarring. J Plast Reconstr Aesthet Surg. 2010;63(6):1022-1029. Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61(9):1049-1058. Brown B, McKenna S, Solomon M, Wilburn J, McGrouther DA, Bayat A. The patient-reported impact of scars measure: development and validation. Plast Reconstr Surg. 2010;125(5):1439-1449. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1-2):113-125. 222 WOUNDS® www.woundsresearch.com 3. 4. 5. 6. 7. 8. 9. 10. Bayat et al 1991;3(5):158–170. Baker LL, Rubayi S, Villar F, Demuth SK. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Rep Regen. 1996;4(1):21-28 25. Colthurst J, Giddings P. A retrospective case note review of the Fenzian electrostimulation system: a novel non-invasive, non-pharmacological treatment. The Pain Clinic. 2007;19(1):7-14. 26. Sebastian A, Syed F, McGrouther DA, Colthurst J, Paus R, Bayat A. A novel in vitro assay for electrophysiological research on human skin fibroblasts: degenerate electrical waves downregulate collagen I expression in keloid fibroblasts. Exp Dermatol. 2011;20(1):64-68. 27. Perry D, Colthurst J, Giddings P, McGrouther DA, Morris J, Bayat A. Treatment of symptomatic abnormal skin scars with electrical stimulation. J Wound Care. 2010;19(10):447-453. 28. Sebastian A, Syed F, Perry D, et al. Acceleration of cutaneous healing by electrical stimulation: degenerate electrical waveform down-regulates inflammation, upregulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Rep Regen. 2011;19(6):693-708. 29. Emtestam L, Kuzmina N, Talme T. Evaluation of the effects of topical clobetasol propionate by visual score, electrical impedance and laser Doppler flowmetry. Skin Res Technol. 2007;13(1):73-78. 30. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961. 31. Moncrieff M, Cotton S, Claridge E, Hall P. Spectrophotometric intracutaneous analysis: a new technique for imaging pigmented skin lesions. Br J Dermatol. 2002;146(3):448-457. 32. Cotton S. A non-invasive imaging system for assisting in the diagnosis of Malignant Melanoma. Birmingham: University of Birmingham; 1998. 33. Farrar JT, Young JP. Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. 34. Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502-507. 35. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, A TE 24. IC Froelich K, Staudenmaier R, Kleinsasser N, Hagen R.Therapy of auricular keloids: a review of different treatment modalities and proposal for a therapeutic algorithm. Eur Arch Otorhinolaryngol. 2007;264(12):1497-1508. 12. Suh H, Petrofsky J, Fish A, et al. A new electrode design to improve outcomes in the treatment of chronic nonhealing wounds in diabetes. Diabetes Technol Ther. 2009;11(5):315-322. 13. Petrofsky J, Lawson D, Prowse M, Suh HJ. Effects of a 2-, 3- and 4-electrode stimulator design on current dispersion on the surface and into the limb during electrical stimulation in controls and patients with wounds. J Med Eng Technol. 2008;32(6):485-497. 14. Jünger M, Arnold A, Zuder D, Stahl HW, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): a prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008;16(4):480-487. 15.Janković A, Binić I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch Dermatol Res. 2008;300(7):377-383. 16. Lee BY, Wendell K, Al-Waili N, Butler G. Ultra-low microcurrent therapy: a novel approach for treatment of chronic resistant wounds. Adv Ther. 2007;24(6):12021209. 17. Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Med Sci Monit. 2007;13(6):CR258-263. 18. Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil. 2001;82(6):721-725. 19. Thuraisingam T, Xu YZ, Eadie K, et al. MAPKAPK-2 signaling is critical for cutaneous wound healing. J Invest Dermatol. 2010;130(1):278-286. 20. Carley PJ, Wainapel SF. Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil. 1985;66(7):443-446. 21. Gault WR, Gatens PF Jr. Use of low intensity direct current in management of ischemic skin ulcers. Phys Ther. 1976;56(3):265-269. 22. Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71(9):639-649. 23. Gentzkow GD, Pollack SV, Kloth LC, Stubbs HA. Improved healing of pressure ulcers using dermapulse, a new electrical stimulation device. WOUNDS. D O N O T D U PL 11. Vol. 25, No. 8 August 2013 223 Bayat et al IC A TE stimulation. Pain. 1978;5(1):31-41. 49. Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271(20):1609-1614. 50. Sebastian A, Allan E, Allan D, Colthurst J, Bayat A. Addition of novel degenerate electrical waveform stimulation with photodynamic therapy significantly enhances its cytotoxic effect in keloid fibroblasts: first report of a potential combination therapy. J Dermatol Sci. 2011;64(3):174-184. D O N O T D U PL and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073-1093. 36. Gilcreast DM, Stotts NA, Froelicher ES, Baker LL, Moss KM. Effect of electrical stimulation on foot skin perfusion in persons with or at risk for diabetic foot ulcers. Wound Rep Regen. 1998:6(5):434-441. 37. Cramp AF, Gilsenan C, Lowe AS, Walsh DM. The effect of high- and low-frequency transcutaneous electrical nerve stimulation upon cutaneous blood flow and skin temperature in healthy subjects. Clin Physiol. 2000:20(2):150-157. 38. Almalty AM, Petrofsky JS, Al-Naami B, Al-Nabulsi J. An effective method for skin blood flow measurement using local heat combined with electrical stimulation. J Med Eng Technol. 2009:33(8):663–669. 39. Dusch M, Schley M, Rukwied R, Schmelz M. Rapid flare development evoked by current frequency-dependent stimulation analyzed by full-field laser perfusion imaging. Neuroreport. 2007:18(11):1101-1105. 40. Cooper CB, Boscardin WJ, Colthurst JR, Kleerup EC. Treatment of mild persistent asthma by cutaneous electronic stimulation. Eur Respir J. 2009:34(2):515-517. 41. Watson T. Electrical stimulation for wound healing: a review of current knowledge. In: Kitchen S, Bazin S, eds. Electrotherapy: Evidence-Based Practice. 11th ed. Edinburgh, Scotland: Churchill Livingstone; 2002:313-34. 42. Salemi S, Aeschlimann A, Reisch N, et al. Detection of kappa and delta opioid receptors in skin--outside the nervous system. Biochem Biophys Res Commun. 2005;338(2):1012-1017. 43. Scott JR, Muangman PR, Tamura RN, et al. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 2005;115(4):1095-1102. 44. McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15(12):497-501. 45. Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002;15(2):119-126. 46. Jagadeesan J, Bayat A. Transforming growth factor beta (TGF-β) and keloid disease. Int J Surg. 2007;5(4):278285. 47. Ramadan A, Elsaidy M, Zyada R. Effect of low-intensity direct current on the healing of chronic wounds: a literature review. J Wound Care. 2008:17(7):292-296. 48. Thosteinsson G, Stonnington HH, Stillwell GK, Elveback LR. The placebo effect of transcutaneous electrical 224 WOUNDS® www.woundsresearch.com