Congenital hypothyroidism Congenital syphilis Dentinogenesis
Transcription
Congenital hypothyroidism Congenital syphilis Dentinogenesis
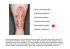
Congenital hypothyroidism Congenital syphilis Dentinogenesis imperfecta Neonatal hyperbilirubinemia Williams syndrome Green primary dentition is visible, predominantly over the incisal two thirds of the anterior teeth. Permanent green pigmentation of primary dentition can result from neonatal hyperbilirubinemia that is classically seen in biliary atresia. Other causes include hemolytic disease and cholestasis caused by severe neonatal sepsis, as in this infant. Big tongue (cretinism) Rhagades (syphilis) Neonatal hyperbilirubinemia Dentinogenesis imperfecta Williams‘ Syndrome Fluorosis and tetracycline Porphyria Das Williams-Beuren-Syndrom (WBS), auch bekannt unter den Synonymen Williams-Syndrom, Fanconi-Schlesinger-Syndrom, idiopathische Hyperkalzämie oder Elfin-face-Syndrom, ist eine genetisch bedingte Besonderheit, deren Ursache in einem Stückverlust (= Deletion) auf dem Chromosom 7 liegt. Das Syndrom wurde nach dem in Auckland tätigen britischen Kardiologen J. C. P. Williams und dem Göttinger Kardiologen Alois J. Beuren benannt. Das WBS wurde in der medizinischen Literatur 1961 von Williams et al. und 1962 von Beuren et al. erstmals ausführlich beschrieben, wobei auch Hinweise vorliegen, dass bereits John Langdon-Down, der das nach ihm benannte Down-Syndrom (Trisomie 21) 1866 und das Prader-Willi-Syndrom erstmals unter wissenschaftlichen Gesichtspunkten beschrieb, eine Beschreibung des WBS vornahm. Kardiovaskuläre Veränderungen/Herzfehler (oft supravalvuläre Aortenstenose (SVAS)/ Verengung der Hauptschlagader unmittelbar am Anschluss an das Herz mit variablem Schweregrad, hypoplastische Aorta/zu schmale Hauptschlagader, Septumfehlbildungen/ Löcher in der Herzscheidewand) bei 90 % der Menschen. Pulmonalstenosen Primäre Nierenfehlbildungen (besondere Lage der Niere, Verengungen/Stenosen an den Nierengefäßen, unterentwickelte Niere, einseitiges Fehlen einer Niere, Hufeisennieren) bei etwa 18 % der Menschen. HIV oder auch bezeichnet als Menschliches Immunschwäche-Virus oder Menschliches Immundefekt-Virus, ist ein Virus, das zur Familie der Retroviren und zur Gattung der Lentiviren gehört. Eine unbehandelte HIV-Infektion führt nach einer unterschiedlich langen, meist mehrjährigen Inkubationszeit in der Regel zu AIDS (engl. acquired immunodeficiency syndrome‚ erworbenes Immundefizienzsyndrom‘). Die Verbreitung von HIV hat sich in den letzten 30 Jahren zu einer Pandemie entwickelt, die nach Schätzungen der Organisation UNAIDS bisher etwa 28 Millionen Leben gefordert hat. Etwa 33,3 Millionen Menschen sind weltweit mit dem Virus infiziert, wobei die Verteilung auf beide Geschlechter in etwa gleich ist. Die Zahl der Neuinfektionen sinkt seit 1997 stetig und lag 2009 bei 2,6 Millionen Menschen. In Deutschland leben gemäß Schätzungen des Robert Koch-Instituts etwa 73.000 Menschen mit HIV, davon 11.500 Frauen und 200 Kinder. CCR5 Tenofovir ist ein Virostatikum, das als Arzneistoff in der Behandlung von HIV-1-Infektionen und Hepatitis B verwendet wird. Tenofovir gehört zur Gruppe der nukleotidischen ReverseTranskriptase-Inhibitoren (NtRTI), die klinisch wie die nukleosidischen Reverse-TranskriptaseInhibitoren (NRTI) eingesetzt werden. Chemisch handelt es sich um ein Nukleotid-Analogon, das aufgrund seiner strukturellen Verwandtschaft mit dem natürlichen Substrat an dessen Stelle in die Virus-DNA eingebaut wird und in Folge die Vermehrung der Viren hemmt. Emtricitabin ist ein chemisches Analogon des Nukleosids Cytidin. Es ist ein Virustatikum aus der Gruppe der NukleosidReverse-Transkriptase-Inhibitoren (NRTI) und wird als Arzneistoff (Coviracil, Triangle Pharmaceutics) zur Behandlung von mit HIV-1 und 2 infizierten Patienten im Rahmen einer antiretroviralen Kombinationstherapie eingesetzt. Es ist ebenfalls gegen HBV wirksam. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women Antiretroviral preexposure prophylaxis is a promising approach for preventing human immunodeficiency virus type 1 (HIV-1) infection in heterosexual populations. We conducted a randomized trial of oral antiretroviral therapy for use as preexposure prophylaxis among HIV-1– serodiscordant heterosexual couples from Kenya and Uganda. The HIV-1–seronegative partner in each couple was randomly assigned to one of three study regimens — once-daily tenofovir (TDF), combination tenofovir–emtricitabine (TDF–FTC), or matching placebo — and followed monthly for up to 36 months. At enrollment, the HIV-1–seropositive partners were not eligible for antiretroviral therapy, according to national guidelines. All couples received standard HIV-1 treatment and prevention services. Oral TDF and TDF–FTC both protect against HIV-1 infection in heterosexual men and women. (Funded by the Bill and Melinda Gates Foundation; Partners PrEP ClinicalTrials.gov number. Partners HealthCare is a non-profit organization that owns several hospitals in Massachusetts, primarily in the Boston area. Massachusetts General Hospital and Brigham and Women's Hospital founded the organization in 1994. Partners is the largest healthcare provider in Massachusetts and many of its hospitals are teaching affiliates of Harvard Medical School. Brigham and Women's Hospital Massachusetts General Hospital Newton-Wellesley Hospital North Shore Medical Center Faulkner Hospital McLean Hospital Martha's Vineyard Community Hospital Nantucket Cottage Hospital Massachusetts General Physician Organization Partners Continuing Care PCHI Partners International Medical Services (PIMS) is a subsidiary of Partners HealthCare that focuses on the advancement of global health. PIMS collaborates with foreign embassies, ministries of health, and universities overseas to improve health status indicators directly through patient care initiatives and indirectly through medical conferences and other educational programs for physicians and nurses. Preexposure Prophylaxis for HIV Infection among African Women Preexposure prophylaxis with antiretroviral drugs has been effective in the prevention of human immunodeficiency virus (HIV) infection in some trials but not in others. In this randomized, double-blind, placebo-controlled trial, we assigned 2120 HIV-negative women in Kenya, South Africa, and Tanzania to receive either a combination of tenofovir disoproxil fumarate and emtricitabine (TDF–FTC) or placebo once daily. The primary objective was to assess the effectiveness of TDF–FTC in preventing HIV acquisition and to evaluate safety. Prophylaxis with TDF–FTC did not significantly reduce the rate of HIV infection and was associated with increased rates of side effects, as compared with placebo. Despite substantial counseling efforts, drug adherence appeared to be low. (Supported by the U.S. Agency for International Development and others Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana Reexposure prophylaxis with antiretroviral agents has been shown to reduce the transmission of human immunodeficiency virus (HIV) among men who have sex with men; however, the efficacy among heterosexuals is uncertain. We randomly assigned HIV-seronegative men and women to receive either tenofovir disoproxil fumarate and emtricitabine (TDF–FTC) or matching placebo once daily. Monthly study visits were scheduled, and participants received a comprehensive package of prevention services, including HIV testing, counseling on adherence to medication, management of sexually transmitted infections, monitoring for adverse events, and individualized counseling on risk reduction; bone mineral density testing was performed semiannually in a subgroup of participants. Botswana has the world's second highest prevalence of HIV infection, estimated in 2008 to be 17.6% overall and approximately 40% among adults 30 to 44 years of age. Daily TDF–FTC prophylaxis prevented HIV infection in sexually active heterosexual adults. The long-term safety of daily TDF–FTC prophylaxis, including the effect on bone mineral density, remains unknown. Brustkrebs ist der häufigste bösartige Tumor der Brustdrüse des Menschen. Er kommt hauptsächlich bei Frauen vor; nur etwa jede hundertste dieser Krebserkrankungen tritt bei Männern auf.[1] In den westlichen Staaten ist Brustkrebs die häufigste Krebsart bei Frauen. Am Brustkrebs sterben mehr Frauen als an irgendeiner anderen Krebserkrankung. Die meisten Erkrankungen treten sporadisch (zufällig) auf, es gibt aber sowohl erbliche als auch erworbene Risikofaktoren. Neben der Heilung sind der Erhalt der betreffenden Brust und vor allem der Lebensqualität erklärtes Ziel der medizinischen Behandlung. Die Therapie besteht in der Regel in einer an das Erkrankungsstadium angepassten Kombination aus Operation sowie Zytostatika-, Hormon- und Strahlentherapie. Neue Ansätze aus dem Gebiet der Krebsimmuntherapie werden außerdem durch monoklonale Antikörper ermöglicht. Das medizinische Vorgehen basiert in hohem Maß auf Erfahrungen aus Studien und ist in weltweit akzeptierten Leitlinien standardisiert. Zahlreiche nationale und internationale Programme zur Früherkennung und zur strukturierten Behandlung sollen die Mortalität (Sterblichkeit) künftig senken. Der Östrogenrezeptor- und Progesteronrezeptorstatus (ER- und PgR-Expression) wird ebenfalls histologisch, genauer immunhistologisch untersucht. Man bestimmt den Prozentsatz derjenigen Tumorzellen, an denen sich die Rezeptoren nachweisen lassen und errechnet aus Prozentsatz und der Färbeintensität einen 12-stufigen Immunreaktiven Score (IRS), oder den international gebräuchlicheren 8-stufigen Allred-Score. Ist die Patientin postmenopausal, erhält sie für in der Regel fünf Jahre entweder Tamoxifen oder einen Aromatasehemmer, welcher durch eine Enzymblockade die Bildung von Östrogen im Muskel- und Fettgewebe unterbindet. Neuere Studienergebnisse deuten an, dass die Aromatasehemmer wirksamer sind als das Tamoxifen, das heißt, die krankheitsfreie Überlebenszeit steigt an. In Studien wird der Aromatasehemmer manchmal sofort verwendet (upfront), in der Regelbehandlung erst nach zwei bis drei Jahren unter Tamoxifen (switch, dt. ‚Wechsel‘), oder nach fünf Jahren (extended). Anastrozole (INN) (marketed under the trade name Arimidex by AstraZeneca) is an aromataseinhibiting drug approved for treatment of breast cancer after surgery, as well as for metastasis in both pre and post-menopausal women. The severity of breast cancer is increased by estrogen, as sex hormones cause hyperplasia, and differentiation at estrogen receptor sites.[3] Anastrozole works by inhibiting the synthesis of estrogen. The patent on Arimidex by AstraZeneca expired 27 June 2010. Anastrozole binds reversibly to the aromatase enzyme through competitive inhibition, inhibits the conversion of androgens to estrogens in peripheral tissues (outside the CNS), and a few CNS sites in various regions within the brain. Fulvestrant (Faslodex, AstraZeneca) is a drug treatment of hormone receptorpositive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. It is an estrogen receptor antagonist with no agonist effects, which works both by down-regulating and by degrading the estrogen receptor. Combination Anastrozole and Fulvestrant in Metastatic Breast Cancer The aromatase inhibitor anastrozole inhibits estrogen synthesis. Fulvestrant binds and accelerates degradation of estrogen receptors. We hypothesized that these two agents in combination might be more effective than anastrozole alone in patients with hormone-receptor (HR)–positive metastatic breast cancer. Postmenopausal women with previously untreated metastatic disease were randomly assigned, in a 1:1 ratio, to receive either 1 mg of anastrozole orally every day (group 1), with crossover to fulvestrant alone strongly encouraged if the disease progressed, or anastrozole and fulvestrant in combination (group 2). Patients were stratified according to prior or no prior receipt of adjuvant tamoxifen therapy. Fulvestrant was administered intramuscularly at a dose of 500 mg on day 1 and 250 mg on days 14 and 28 and monthly thereafter. The primary end point was progression-free survival, with overall survival designated as a prespecified secondary outcome. Toxic effects of grade 3 or higher were observed in 42 patients who received anastrozole alone (12.7%) and in 51 patients who received combination therapy (14.7%) (P=0.44). The most common grade 3 toxic effects were musculoskeletal pain (2.8%), influenza-like symptoms (2.4%), gastrointestinal disturbances (1.5%), and hematologic effects (1.5%). The combination of anastrozole and fulvestrant was superior to anastrozole alone or sequential anastrozole and fulvestrant for the treatment of HR-positive metastatic breast cancer, despite the use of a dose of fulvestrant that was below the current standard. We suggest that trials of adjuvant therapy should be performed in which the combination of an aromatase inhibitor and high-dose fulvestrant is compared with an aromatase inhibitor alone or high-dose fulvestrant alone, in patients with estrogen-receptor–positive tumors for whom chemotherapy is not necessary. A 30-year-old woman with a history of bulimia presented to the emergency department after swallowing a knife. Her husband later disclosed that 4 years earlier she had swallowed a knife that required surgical removal with exploratory laparotomy. Consultation with a psychiatrist was recommended, and the patient was later transferred to an inpatient psychiatric unit. Breathtaking Journey A 48-year-old man came to the emergency department in early August with a 3-day history of influenza-like symptoms and profound dyspnea on exertion, which had started 3 days after his return to Boston from a vacation in California. On his return flight, subjective fevers, headache, myalgias, and nausea developed, and the patient had one episode of vomiting. Over the next 2 days, a nonproductive cough and profound exertional dyspnea developed. The patient said that he did not have a rash, neck stiffness, visual changes, diarrhea, dysuria, or joint pain. Three weeks before admission, the patient hiked and camped in the Catskill Mountains for 9 days. On the first night, he slept on the floor of a lean-to, where he observed mice. Two weeks before admission, he removed an attached tick. One week before admission, he flew to California and spent 4 days hiking and camping in the San Joaquin Valley. There was no rash. The mucous membranes were dry. The neck was supple, with no meningeal signs. No peripheral adenopathy was noted. The jugular venous pressure was less than 5 cm of water. Chest auscultation revealed crackles in the lower lobes of both lungs; the heart tones were regular, with normal carotid upstrokes. The abdomen was soft and nontender. There was no hepatosplenomegaly. There were no joint effusions or peripheral edema. The neurologic examination was unremarkable. he patient was empirically treated with intravenous fluids, ceftriaxone, and doxycycline. He remained afebrile, but during the first 24 hours after admission, hypoxemia developed while the patient was breathing ambient air at rest; supplemental oxygen at a rate of 3 liters per minute was added. On the third hospital day, the white-cell count was 7400 per cubic millimeter, with 48% neutrophils, 11% band forms, 11% monocytes, 12% lymphocytes, 16% atypical lymphocytes, and 2% metamyelocytes. The hematocrit was 46%, and the platelet count 59,000 per cubic millimeter. The sodium level was 136 mmol per liter, alanine aminotransferase 224 U per liter, aspartate aminotransferase 212 U per liter, total bilirubin 0.9 mg per deciliter (15 µmol per liter), and alkaline phosphatase 58 U per liter. Serologic studies sent on admission were returned after the patient had been discharged. Testing for Rocky Mountain spotted fever, B. microti, A. phagocytophilum, Ehrlichia chaffeensis, C. immitis, and leptospira was negative. Serologic testing for IgG antibody to mycoplasma showed an immune status ratio of 0.91 to 1.09 (equivocal range). Testing with the use of an enzyme-linked immunosorbent assay (ELISA) for IgG and IgM antibodies specific to the Sin Nombre hantavirus group was positive, at levels of 2.35 and 8.22, respectively (normal value, <1.10). Die Gattung Hantavirus aus der Familie der Bunyaviridae umfasst unter anderem die humanpathogenen Arten Hantaan-Virus, Puumala-Virus, Dobrava-Belgrad-Virus, Seoul-Virus, Korea-Fieber-Virus, Sin-Nombre-Virus. Diese behüllten Einzel-Strang(−)-RNA-Viren [ss(−)RNA] verursachen Lungenerkrankungen, akutes Nierenversagen (Nephrotisches Syndrom) oder schwere hämorrhagische Fiebererkrankungen insbesondere im südasiatischen Raum, aber auch in Europa, siehe: Vorkommen. Der Name Hanta geht auf den Fluss Hantan in Südkorea zurück, an welchem in den 1950er-Jahren während des Koreakrieges mehr als 3000 amerikanische Soldaten an einem ungewöhnlich starken Fieber mit anschließend häufigen Nierenversagen erkrankten. Erst 1977 gelang es durch Ho Wang Lee und andere, das bis dahin unbekannte Virus zu isolieren. Die Übertragung geschieht durch verschiedene Nager, die mit dem Speichel, den Fäkalien und dem Urin (Virurie) große Mengen an Erregern ausscheiden. Bei den Nagern sind vor allem Mäuse, in Deutschland besonders die Rötelmaus als Überträger festgestellt, die jedoch selbst nicht erkranken, auch wenn sie, einmal infiziert, lebenslang infektiös bleiben Linagliptin (Trajenta®) ist ein Arzneistoff zur peroralen Behandlung von Typ II-Diabetes. Im August 2011 erteilte die Europäische Kommission die Zulassung für das von dem pharmazeutischen Unternehmen Boehringer Ingelheim entwickelte Medikament. Linagliptin ist ein Wirkstoff aus der Gruppe der Dipeptidylpeptidase-4-Inhibitoren. Diese hemmen das Enzym Dipeptidylpeptidase 4 (DPP-4). Linagliptin ist ein Inhibitor des Dipeptylpeptidase-Isoenzyms DPP-4, welches er kompetitiv und selektiv gegenüber anderen Isozenzymen, wie die Dipeptylpeptidasen DPP-8 und DPP-9, hemmt. Als Dipeptylpeptidase-4-Inhibitor hemmt es den Abbau des Inkretin-Hormons Glucagon-like-peptide 1 (GLP-1). Im Vergleich zu den bereits kommerziell genutzten Gliptinen Sitagliptin, Saxagliptin und Vildagliptin zeichnet sich Linagliptin zumindest experimentell durch eine höhere Wirkpotenz und eine längere Wirkdauer aus. Das Protein Glucagon-like Peptide 1 (GLP-1) ist ein Peptidhormon, das eine wichtige Rolle im Zuckerstoffwechsel im Rahmen des Inkretin-Effekts – der Insulinantwort der βZellen in der Bauchspeicheldrüse auf Glucosezufuhr über den Darm – spielt. Erstmals wurde das GLP-1 im Jahre 1979 von der Arbeitsgruppe um Prof. Werner Creutzfeldt an der Universität Göttingen beschrieben. GLP-1 wird als Darmhormon von den neuroendokrinen L-Zellen des Darms produziert und bei Nahrungsaufnahme in den Blutkreis freigesetzt. Es wird innerhalb von Minuten von dem Enzym Dipeptidylpeptidase 4 (DPP 4) abgebaut und muss also ständig neu produziert werden. Glimepirid ist ein orales Antidiabetikum aus der Stoffgruppe der Sulfonylharnstoffe, welches direkt in den β-Zellen der Bauchspeicheldrüse die Insulinfreisetzung steigert. Der Wirkstoff ist nicht mehr patentgeschützt und es sind verschiedene Generika verfügbar. Da es für den Wirkungseintritt nötig ist, dass der Körper zumindest eingeschränkt selbst Insulin produziert, kann Glimepirid nicht zur Behandlung des Diabetes mellitus Typ 1 eingesetzt werden. Arzneimittel, die Glimepirid enthalten, sind verschreibungspflichtig. Binding closes the linked ATPsensitive potassium channels, which leads to decreased potassium influx and subsequent depolarization of the beta-cell membrane. Voltage-dependent calcium channels open and result in an influx of calcium, causing translocation and exocytosis of secretory granules of insulin to the cell surface 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial Addition of a sulphonylurea to metformin improves glycaemic control in type 2 diabetes, but is associated with hypoglycaemia and weight gain. We aimed to compare a dipeptidyl peptidase-4 inhibitor (linagliptin) against a commonly used sulphonylurea (glimepiride). In this 2-year, parallelgroup, non-inferiority double-blind trial, outpatients with type 2 diabetes and glycated haemoglobin A1c (HbA1c) 6·5—10·0% on stable metformin alone or with one additional oral antidiabetic drug (washed out during screening) were randomly assigned (1:1) by computer-generated random sequence via a voice or web response system to linagliptin (5 mg) or glimepiride (1—4 mg) orally once daily. Study investigators and participants were masked to treatment assignment. The primary endpoint was change in HbA1c from baseline to week 104. Analyses included all patients randomly assigned to treatment groups who received at least one dose of treatment, had a baseline HbA1c measurement, and had at least one on-treatment HbA1c measurement. The overall incidence of hypoglycaemic events was, significantly, 4·8-times lower with linagliptin than with glimepiride (58 [7%] of 776 vs 280 [36%] of 775 patients; Bodyweight decreased with linagliptin (—1·4 [SE 0·2] kg) but increased with glimepiride (1·3 [0·2] kg Our findings support the use of linagliptin in combination with metformin as a therapeutic option for treatment of type 2 diabetes. This study adds to the evidence base for evaluation of recommendations for second-line treatment after metformin. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial Acute pyelonephritis is a common infection in adult women, but there is a paucity of controlled trials of its treatment and the optimum duration of antibiotic treatment has not been properly defined. We compared the efficacy of ciprofloxacin for 7 days and 14 days in women with community-acquired acute pyelonephritis. In a prospective, non-inferiority trial undertaken at 21 centres of infectious diseases in Sweden, women (aged ≥18 years) who were not pregnant and had a presumptive diagnosis of acute pyelonephritis were randomly assigned to oral treatment with ciprofloxacin 500 mg twice daily for 7 days or 14 days. The first week was open label. A computer-generated randomisation list in block sizes of two was used for treatment allocation in a 1:1 ratio. The study was double-blind and placebo-controlled during the second week of treatment, which was either continuation of ciprofloxacin 500 mg or placebo tablets twice daily according to the randomisation code. Patients, carers, site investigators, and trial coordinating centre staff were masked to group assignment. The primary endpoint was the clinical and bacteriological outcome 10—14 days after completion of treatment with active drug. Eligible patients had fever of at least 38·0°C (measured at home or in the emergency department) and at least one symptom or sign relating to the urinary tract such as flank pain, costovertebral angle tenderness, dysuria, urgency, or frequency. Our results show that communityacquired acute pyelonephritis in women can be treated successfully and safely with oral ciprofloxacin for 7 days and even in older patients and those with a more severe infection An otherwise healthy 45-year-old woman presented with a 6-month history of gradual visual loss in both eyes. Her best-corrected visual acuity was 20/30 in both eyes. Slitlamp examination showed bilateral —predominantly peripheral—corneal deposits of numerous minute, crystalline materials. The remainder of the ocular examination was unremarkable. A clinical diagnosis of monoclonal gammopathy was made on the basis of shimmering and polychromatic corneal deposits. Serum IgG concentration was 30·3 g/L (normal 8·1—16·9 g/L) and serum electrophoresis showed a monoclonal gammopathy involving IgG-kappa light chain. Our patient was referred to a haematologist and IgG-kappa multiple myeloma was diagnosed by bone marrow biopsy. Treatment was initiated with thalidomide, and no ocular-specific drugs were prescribed. During the 7-year follow-up, while maintained on a low-dose thalidomide, our patient was regularly followed up and her vision remained stable. Although still present, the amount of these corneal crystalline deposits appeared to be decreasing and migrate away from the peripheral corneas. The effect of mass immunisation campaigns and new oral poliovirus vaccines on the incidence of poliomyelitis in Pakistan and Afghanistan, 2001–11: a retrospective analysis Pakistan and Afghanistan are two of the three remaining countries yet to interrupt wild-type poliovirus transmission. The increasing incidence of poliomyelitis in these countries during 2010—11 led the Executive Board of WHO in January, 2012, to declare polio eradication a “programmatic emergency for global public health”. We aimed to establish why incidence is rising in these countries despite programme innovations including the introduction of new vaccines. We did a matched case-control analysis based on a database of 46 977 children aged 0—14 years with onset of acute flaccid paralysis between Jan 1, 2001, and Dec 31, 2011. The vaccination history of children with poliomyelitis was compared with that of children with acute flaccid paralysis due to other causes to estimate the clinical effectiveness of oral poliovirus vaccines (OPVs) in Afghanistan and Pakistan by conditional logistic regression. We estimated vaccine coverage and serotype-specific vaccine-induced population immunity in children aged 0—2 years and assessed their association with the incidence of poliomyelitis over time in seven regions of Afghanistan and Pakistan. The effectiveness of bivalent OPV is comparable with monovalent OPV and can therefore be used in eradicating serotype 1 poliomyelitis whilst minimising the risks of serotype 3 outbreaks. However, decreases in vaccination coverage in parts of Pakistan and southern Afghanistan have severely limited the effect of this vaccine. Vaccination coverage decreased during 2006— 11 in the Federally Administered Tribal Areas (FATA), Balochistan, and Khyber Pakhtunkhwa in Pakistan and in southern Afghanistan. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study Although CT scans are very useful clinically, potential cancer risks exist from associated ionising radiation, in particular for children who are more radiosensitive than adults. We aimed to assess the excess risk of leukaemia and brain tumours after CT scans in a cohort of children and young adults. In our retrospective cohort study, we included patients without previous cancer diagnoses who were first examined with CT in National Health Service (NHS) centres in England, Wales, or Scotland (Great Britain) between 1985 and 2002, when they were younger than 22 years of age. We obtained data for cancer incidence, mortality, and loss to follow-up from the NHS Central Registry from Jan 1, 1985, to Dec 31, 2008. We estimated absorbed brain and red bone marrow doses per CT scan in mGy and assessed excess incidence of leukaemia and brain tumours cancer with Poisson relative risk models. To avoid inclusion of CT scans related to cancer diagnosis, follow-up for leukaemia began 2 years after the first CT and for brain tumours 5 years after the first CT. Use of CT scans in children to deliver cumulative doses of about 50 mGy might almost triple the risk of leukaemia and doses of about 60 mGy might triple the risk of brain cancer. Tetanus, auch Wundstarrkrampf genannt, ist eine häufig tödlich verlaufende Infektionskrankheit, welche die muskelsteuernden Nervenzellen befällt und durch das Bakterium Clostridium tetani ausgelöst wird. Die resistenten Sporen des Bakteriums kommen nahezu überall vor, auch im Straßenstaub oder in der Gartenerde. Die Infektion erfolgt durch das Eindringen der Sporen in Wunden. Unter anaeroben Bedingungen, d. h. unter Sauerstoff-Abwesenheit, vermehrt sich das Bakterium und sondert Giftstoffe (Toxine) ab. Das proteolytische Toxin Tetanospasmin schädigt die muskelsteuernden Nervenzellen und verursacht dadurch die typischen Lähmungen und Muskelkrämpfe. Das Toxin Tetanolysin ist herzschädigend. Tetanolysin ist hämolysierend und kardiotoxisch, aber für die typischen Symptome der Krankheit unbedeutend. Wichtiger ist Tetanospasmin, das über periphere Nervenbahnen in das Zentralnervensystem gelangt. Dort greift es Proteine (SNARE) an, die zur Freisetzung der Neurotransmitter (Glycin und GABA) der Renshaw-Zellen im Vorderhorn des Rückenmarks nötig sind. Tetanospasmin ist eine Endopeptidase. Die Blockade geschieht über eine enzymatische Inaktivierung des Synaptobrevin/Vesicular Associated Membrane Complex, wodurch die Freisetzung der Neurotransmitter durch Exozytose gestört ist. Damit kommt es zur unkontrollierten Aktivierung der alpha-Motoneuronen und zu tonischen (andauernden) und klonischen (zuckenden) Verkrampfungen der quergestreiften (Willkür-)Muskulatur. Sept. 2011 86-year-old woman was admitted to our medical intensive care unit with trismus and neck stiffness. 2 years earlier, she had been diagnosed with marginal zone lymphoma. Her medications included chlorambucil from January to October, 2010, followed by rituximab, cyclophosphamide, vincristine, and prednisone in March, 2011. Prednisone (20 mg per day) was maintained from March to August, 2011. 5 months before presentation, she had several falls and she received local care with 3 stitches for a chin wound in April 2011, and was given a first tetanus vaccination. She had not previously been vaccinated for tetanus and complete vaccination was proposed, but she refused. In August, 2011, she had another fall, resulting in a severe wound to her left leg. She was given oral amoxicillin clavulanate and daily wound care for a week. She reported 10 days afterwards, dysphagia, gait instability, dysarthria, stiffness of the neck, and intractable trismus. She was referred the same day to our hospital. On presentation, she was alert and fully oriented. Core temperature was 37°C. She was severely malnourished. Her weight was 42 kg, for a height of 152 cm, and she had diffuse muscle atrophy, skin fragility, and splenomegaly. The left calf wound was dry and clean. There was no limb weakness. Plantar responses were normal. The absence of antibodies suggests that our patient was unable to produce an immune response to tetanus. Ageing, active lymphoma, chemotherapy, and rituximab can impair the immune response. For patients with dirty wounds who are elderly, have a haematological malignancy, or are on chemotherapy, a tetanus-specific single-step immunoassay should be done even if previous vaccination is apparently correct. If the assay results are negative, human tetanus immunoglobulins should be given.